Of the more than 38 million Americans who have dry eye disease (DED), approximately 9 in 10 experience evaporative dry eye.1,2 Until recently, however, U.S. eyecare professionals had no proven prescription therapies to directly address the underlying cause of evaporative dry eye: excessive evaporation.3 This situation was remedied last year, when the U.S. Food and Drug Administration approved perfluorohexyloctane (PFHO) ophthalmic solution (Miebo™, Bausch + Lomb), the first and only prescription eye drop that directly targets tear evaporation. (See Full Prescribing Information.)
Miebo is a semifluorinated alkane indicated for treatment of the signs and symptoms of DED.4 It mimics the key functions of natural meibum,4,5 forming an anti-evaporative monolayer at the air-tear interface. As a single-ingredient formulation, Miebo is water-free, preservative-free, and steroid-free. In two pivotal clinical trials, Miebo demonstrated rapid and sustained relief of DED symptoms, as well as excellent tolerability.4
Through a series of webinars hosted by Bausch + Lomb, leading optometrists and ophthalmologists weighed in on this novel therapy, discussing its unique formulation, its performance in clinical trials, and what it will mean for patients who are struggling with DED.
Causes and Consequences of Evaporative DED
“The majority of dry eye disease, about 86%, has an evaporative etiology,” says Jai G. Parekh, MD, MBA, cofounder of EyeCare Consultants of New Jersey. “We haven’t spoken about that much because we haven’t had anything to treat that etiology before now.” 6-8
The causes of excessive tear evaporation run the gamut from lipid, aqueous, and mucin deficiencies to environmental and lifestyle factors.9-17 While lipid deficiency, a consequence of meibomian gland dysfunction (MGD),5 is the leading cause of evaporative DED, “We should not discount the role of aqueous and mucin, which also comprise a healthy tear film,” says Cecelia Koetting, OD, of the University of Colorado in Denver. “For example, with low aqueous volume, tear film integrity may be disrupted rapidly between blinks, even with a normal lipid layer. Once an imbalance develops, excessive evaporation occurs.”
Environmental factors, such as dry, windy, arid climates, as well as air conditioning and forced-hot-air heating systems, are all suspect when patients present with symptoms of DED. Perhaps even more significant are certain behaviors associated with today’s lifestyle: more stress, less sleep, and prolonged use of digital devices.9-10
“As we stare at computer and cell phone screens, and we’re more focused—even reading a book—our blink rate decreases significantly. Reduced and incomplete blinking over time will result in greater evaporative loss and ocular surface dryness,” explains Lisa Nijm, MD, of Warrenville EyeCare & LASIK in Illinois.
When tear evaporation exceeds total tear supply, loss of homeostasis follows.14,19 This multifactorial process triggers a vicious cycle of desiccation stress on the ocular surface; tissue damage; and inflammation. Several proinflammatory cytokines are often found to be elevated in the tears of patients who have DED.20
“The lacrimal output starts to suffer, and inflammatory mediators are elevated in the tear film,” notes Laura M. Periman, MD, of Periman Eye Institute in Seattle. “We know those cytokines increase dramatically, resulting in epithelial cell breakdown. The corneal nerves are exposed and can become dysmorphic, which adds to that vicious cycle.” (Figure 1)
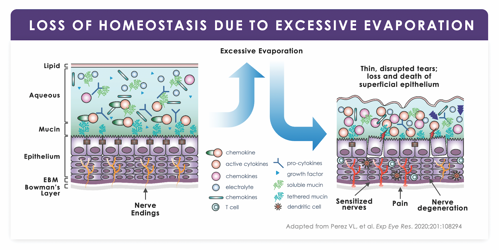
The cycle begets a range of symptoms (fluctuating vision, eye fatigue, tearing, itching, and discharge), not all of which are immediately identifiable as DED—especially to the patient.
“A lot of patients think, ‘Well, yeah, my eyes are a little tired. They’re a little burn-y. They’re a little itchy.’ But they don’t know there’s something that can help them,” says Audrey Rostov, MD, a Seattle-based cornea, cataract, and refractive surgeon. “If they tell you that they have fluctuating vision, then they have some sort of dry eye disease.”
Dr. Nijm points out that patients might not even bring up symptoms of dry eye because they don’t recognize them as such. “As clinicians, we have to be proactive in asking patients for these symptoms,” she says. “In every exam, I try to include questions related to fluctuating vision, eye fatigue, tearing, itching, and discharge. It takes just a few minutes, but it can make all the difference in not missing dry eye cases.”
Mark E. Schaeffer, OD, of MyEyeDr in Birmingham, Ala., stresses the importance of also checking for MGD at every visit. “Every single time I get in the slit lamp, I use a meibomian gland expressor,” he says. “If you’re not pressing on these glands, you could be missing early disease.”
“It’s vital for us to tease out those who have evaporative DED and break the cycle,” reiterates Dr. Parekh. “Artificial tears, anti-inflammatory agents, tear stimulators, and immunomodulators don’t address evaporative disease. Thus, patients’ symptoms persist, and they become discouraged and distressed. I believe targeting evaporation with Miebo™ is going to be a game-changer for us.”
Marc Bloomenstein, OD, of Schwartz Laser Eye Center in Scottsdale, Ariz., agrees. “Everything we do is meant to get our patients as close to homeostasis as possible,” he says. “If we can limit evaporation by using Miebo, we can limit desiccating stress and inflammation.”
“Not only does Miebo address real-time adjustment of the evaporative burden, but it also has downstream benefits for the ocular surface,” adds Dr. Periman.
How Miebo™ Works on the Ocular Surface
When the tear film is unstable, gaps in the lipid layer don’t allow full protection from evaporation (Figure 2). Miebo™ addresses this by forming a stable monolayer at the air-liquid interface, enabled by its polarized molecular structure: an aerophilic fluorinated alkane segment at top, and a lipophilic hydrocarbon segment at bottom.
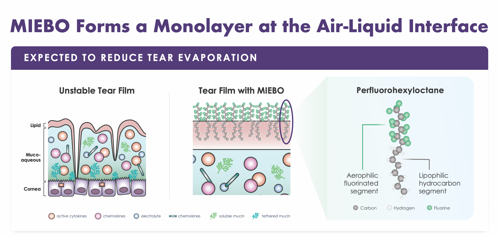
“I love explaining this to patients, because it makes sense to them,” says Dr. Nijm. “I use the example of a buoy: The top part stays in the air and the bottom part stays in the water. When the molecules line up next to each other, they form a barrier that reduces tear evaporation on the surface.”
Dr. Rostov uses another analogy to describe Miebo’s effect. “I’ve had patients say, ‘It feels so silky, so nice,’” she reports. “It coats the ocular surface like a silk blanket.”
Vittitow and colleagues evaluated the inhibitory effect of Miebo versus meibum on the evaporation rate of saline using an in vitro model.21 They found that Miebo was 4x more
effective at inhibiting evaporation than meibum alone, noting that Miebo seems to reduce surface friction and forms a long-lasting, anti-evaporative barrier on the ocular surface, facilitating healing.
“In an earlier rabbit study,22 we saw perfluorohexyloctane stay in the tears for up to 6 hours,” says Dr. Schaeffer. “With an average drop, the majority of it is usually running down the face, and what’s left in the eye dissipates pretty quickly. So, with previous therapeutic drops, we only had a short residence time to try to maximize bioavailability.”
“That’s a very important distinction,” notes Dr. Bloomenstein. “Lubricating drops don’t last. Miebo is still present at the ocular surface after 6 hours. It has some impressive legs.”
“We talk about the shearing force of a blink as the eyelid applies friction on the ocular surface, like a windshield wiper,” says I. Benjamin Gaddie, OD, of Gaddie Eye Centers in Louisville, Ky. “By forming a lubricating layer, Miebo™ may allow that ‘apparatus’ to move more smoothly across the eye with less friction, thus promoting ocular surface healing.”
Agarwal and colleagues evaluated the effect of semifluorinated alkanes on ocular surface and tear fluid dynamics in preclinical models.23 Among their findings, they reported that the low surface tension of PFHO facilitates rapid spreading. They also found that PFHO improved the lipid layer grade significantly after topical application, supporting its potential to stabilize the tear film and thus provide symptomatic relief from evaporative dry eye disease.
Results from these and other studies led to the design of two large phase 3 trials to evaluate the safety and efficacy of Miebo™ for the treatment of DED.
Key Findings from Phase 3 Trials
A total of 1,217 patients, all of whom had DED and clinical signs of MGD, were enrolled in the GOBI (n=597) and MOJAVE (n=620) multicenter, double-masked clinical trials.4 Patients were randomly assigned 1:1 to receive Miebo or hypotonic saline (control) qid. Outcome measures were change from baseline in total corneal fluorescein staining (tCFS) and change from baseline in visual analog scale (VAS) dryness scores at days 15 (secondary) and 57 (primary).
“It’s important to examine how studies are structured—the inclusion and exclusion criteria, for example—to assess the validity and utility of the data,” Dr. Koetting says. “To participate in the GOBI and MOJAVE studies, patients had to have greater than 6 months of self-reported history of DED. They also had to have tCFS scores between 4 and 11, and total MGD scores equal to or greater than 3. In my practice, these would be patients with mild to moderate disease.”
Equally important are the exclusion criteria to eliminate factors that might compromise outcomes. In these trials, patients were excluded if they had active blepharitis that required treatment, wore contact lenses, or had a recent history of punctal plugs or MGD procedures. Also excluded were patients who used topical steroids, other prescription DED medications, serum tears, glaucoma medications, or any type of tear stimulator. Only patients with MGD and evaporative DED were enrolled.
Miebo demonstrated consistent results across the clinical trials.
“There was rapid and sustained improvement in total corneal fluorescein staining as early as day 15 (Figure 3). By day 57, total corneal staining in eyes treated with Miebo had improved twice as much as those treated with hypotonic saline,” Dr. Gaddie notes. “I think cornea specialists would agree, reducing corneal staining is one of the most frustrating and difficult things to do in clinical practice.”

“The two studies are dead-on in their findings,” Dr. Bloomenstein points out. “That mean improvement in tCFS from baseline—30% in the GOBI trial and 33% in the MOJAVE trial—is just really impressive.”
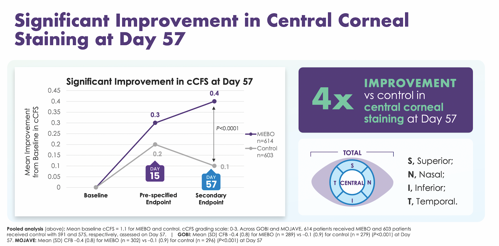
How these outcomes translate to the real world is significant, Dr. Parekh adds. “Corneal staining is commensurate with the quality and the quantity of vision,” he says. “To me, this immediately translates to improvements in visual outcomes for our patients.” Miebo also showed significant improvement in central corneal staining at day 57 (Figure 4).
“While total corneal staining is an important sign of DED, to me, central corneal staining is even more of a culprit when we see poor vision and fluctuating vision,” Dr. Gaddie says. “Of all the trial data reported, I think the outcomes on central corneal staining are most impressive. A 4x improvement in central corneal staining really speaks to what this medication is doing.”
Dr. Nijm agrees. “As a cornea specialist, this data is significant to me. That central cornea is where the majority of your vision is,” she says. “So, to improve the staining there makes a huge impact on my patients and on their satisfaction with treatment.”
“These numbers look small. But when you start out at 1.1, dropping down a half point makes a significant difference,” adds Dr. Schaeffer. “When we talk to our patients about their lives, about their vision being significantly better—that’s because we’ve cleared up central corneal staining or improved it dramatically.”
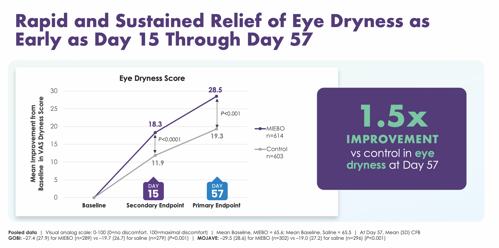
VAS dryness scores showed rapid and sustained relief of eye dryness with Miebo™ as early as day 15 and continued through day 57. These outcomes were consistent and sustained in both GOBI and MOJAVE (Figure 5).
“That’s what matters most to our patients,” Dr. Periman says. “The mean eye dryness scores at baseline were about the same in the Miebo group (65.6) as in the control group (65.5). Those were some uncomfortable eyes! By day 15 of the studies, patients in the Miebo group had an 18.3 point reduction in the eye dryness scores, and by day 57, their scores showed a 28.5 point reduction. That’s a significant decrease, and one of the outcomes that patients most appreciate.”
Dr. Koetting notes, “All of our prescription drops to date have been immunomodulators, tear stimulators, or anti-inflammatory agents, which take time to work. These therapies are not known to relieve dryness quickly, so an eye drop that provides rapid and sustained relief is a welcome addition to our toolkits.”
According to their VAS scores, patients had, on average, a 1.5x improvement in dryness with Miebo compared with control at day 57.
Positive Tolerability Scores Predict Persistence
While patients may be willing, even eager, to try a topical medication to ease their DED symptoms, convincing them to persist with treatment can be a challenge.
Yet in the two pivotal clinical studies, Miebo demonstrated an average discontinuation rate of just 0.2% due to adverse events, and a 0.5% incidence of burning or stinging on instillation. Furthermore, across studies, investigators reported only one ocular adverse event with an incidence of >2% (2.1% of patients experienced blurred vision, which usually resolved within minutes (Figure 6).
“Typically, eye drops burn and sting because of the active molecules, the preservatives, and other ingredients,” Dr. Periman says. “If a patient’s tear osmolarity is already compromised and they instill an isotonic solution, the corneal nerves are going to react. I love the fact that the rate of burning and stinging was so low with Miebo.”
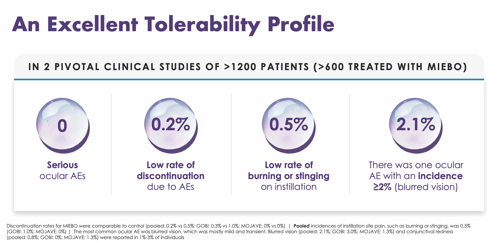
Figure 6. Miebo™ demonstrated excellent tolerability with a low incidence of adverse events across two pivotal clinical trials.
Dr. Bloomenstein concurs. “Think about all the other drops that we’ve prescribed for patients and had to overcome them saying, ‘Oh, it burns.’ We don’t have to do that with Miebo.”
Miebo’s unusually small drop size (11 μL) may contribute to its comfort.
“That small drop of Miebo, which is 100% PFHO, helps ensure a favorable effect because the medication stays on the ocular surface and does not wash itself out,” says Dr. Gaddie.
In the clinical studies, most patients rated Miebo comfortable or very comfortable. Comfort was assessed using a questionnaire given approximately 2 minutes after dosing on day 1 of the GOBI and the MOJAVE studies.4
“Miebo is exceptionally well tolerated, from a medical standpoint,”
says Dr. Parekh. “More than 80% of the patients treated with Miebo gave it a comfort score of 7 or higher, with 10 being the most comfortable.4 Having a drop with that level of comfort on instillation means that patients may stay with the therapy with fewer callbacks.”
Summary
These clinicians, several of whom participated in the FDA clinical trials for Miebo, are convinced that this novel medication that targets evaporation will have a positive impact on their dry eye practices, filling a long-standing treatment gap.
Dr. Periman foresees a synergistic effect. “We now have a medication that is very well tolerated and provides rapid and sustained relief,” she said. “Miebo is a wonderful adjunct to the great tools that we already have for our patients with dry eye disease.”
“I always tell patients, ‘If you don’t have a good tear layer, then you’re not going to have good vision,’” says Dr. Rostov.
REFERENCES
1. Paulsen AJ, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799-806.
2. US population by age and gender, 2020.
3. White DE, Zhao Y, Ogundele A, et al. Real-world treatment patterns of cyclosporine ophthalmic emulsion and lifitegrast ophthalmic solution among patients with dry eye. Clin Ophthalmol. 2019;13:2285-2292.
4. MIEBO™ [package insert]. Bridgewater, NJ: Bausch & Lomb Americas Inc; 2023.
5. Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: Focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12(3):1397-1418.
6. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-283.
7. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478.
8. Badian RA, Utheim TP, Chen X, et al. Meibomian gland dysfunction is highly prevalent among first-time visitors at a Norwegian dry eye specialist clinic. Sci Rep. 2021;11(1):23412.
9. Wolffsohn JS, Wang MTM, Vidal-Rohr M, et al. Demographic and lifestyle risk factors of dry eye disease subtypes: A cross-sectional study. Ocul Surf. 2021;21:58-63.
10. Wang MTM, Muntz A, Mamidi B, Wolffsohn JS, Craig JP. Modifiable lifestyle risk factors for dry eye disease. Cont Lens Anterior Eye. 2021;44(6):101409.
11. Al-Mohtaseb Z, Schachter S, Shen Lee B, Garlich J, Trattler W. The relationship between dry eye disease and digital screen use. Clin Ophthalmol. 2021;15:3811-3820.
12. Covita A, Chen MH, Leahy C. Correlation between meibomian gland appearance and tear breakup time using a slit scanning ophthalmoscope. [Abstract] Invest Ophthalmol Vis Sci. 2019;60(9):6793.
13. Arita R, Minoura I, Morishige N, et al. Development of definitive and reliable grading scales for meibomian gland dysfunction. Am J Ophthalmol. 2016;169:125-137.
14. McMonnies CW. Aqueous deficiency is a contributor to evaporation-related dry eye disease. Eye Vis (Lond). 2020;7:6.
15. Alshammeri S, Madden L, Hagan S, Pearce EI. Strip meniscometry tube: a rapid method for assessing aqueous deficient dry eye. Clin Exp Optom. 2020;103(4):469-473.
16. Kawashima M, Yamada M, Suwaki K, et al. A clinic-based survey of clinical characteristics and practice pattern of dry eye in Japan. Adv Ther. 2017;34(3):732-743.
17. Tsubota K, Yokoi N, Shimazaki J, et al. New perspectives on dry eye definition and diagnosis: A consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65-76.
18. Tsubota K, Nakamori K. Dry eyes and video display terminals. N Engl J Med. 1993;328(8):584.
19. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510.
20. Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28(9):1023-1027.
21. Vittitow J, Kissling R, DeCory H, Borchman D. In vitro inhibition of evaporation with perfluorohexyloctane, an eye drop for dry eye disease. Curr Ther Res Clin Exp. 2023;98:100704.
22. Krösser S, Spencer E, Grillenberger R, Struble CB, Fischer K. Ocular and systemic distribution of 14C-perfluorohexyloctane following topical ocular administration to rabbits. Invest Ophthalmol Vis Sci. 2018;59:2656.
23. Agarwal P, Khun D, Krösser S, et al. Preclinical studies evaluating the effect of semifluorinated alkanes on ocular surface and tear fluid dynamics. Ocul Surf. 2019;17(2):241-249.
© 2024 Bausch + Lomb Incorporated
SPONSORED CONTENT








