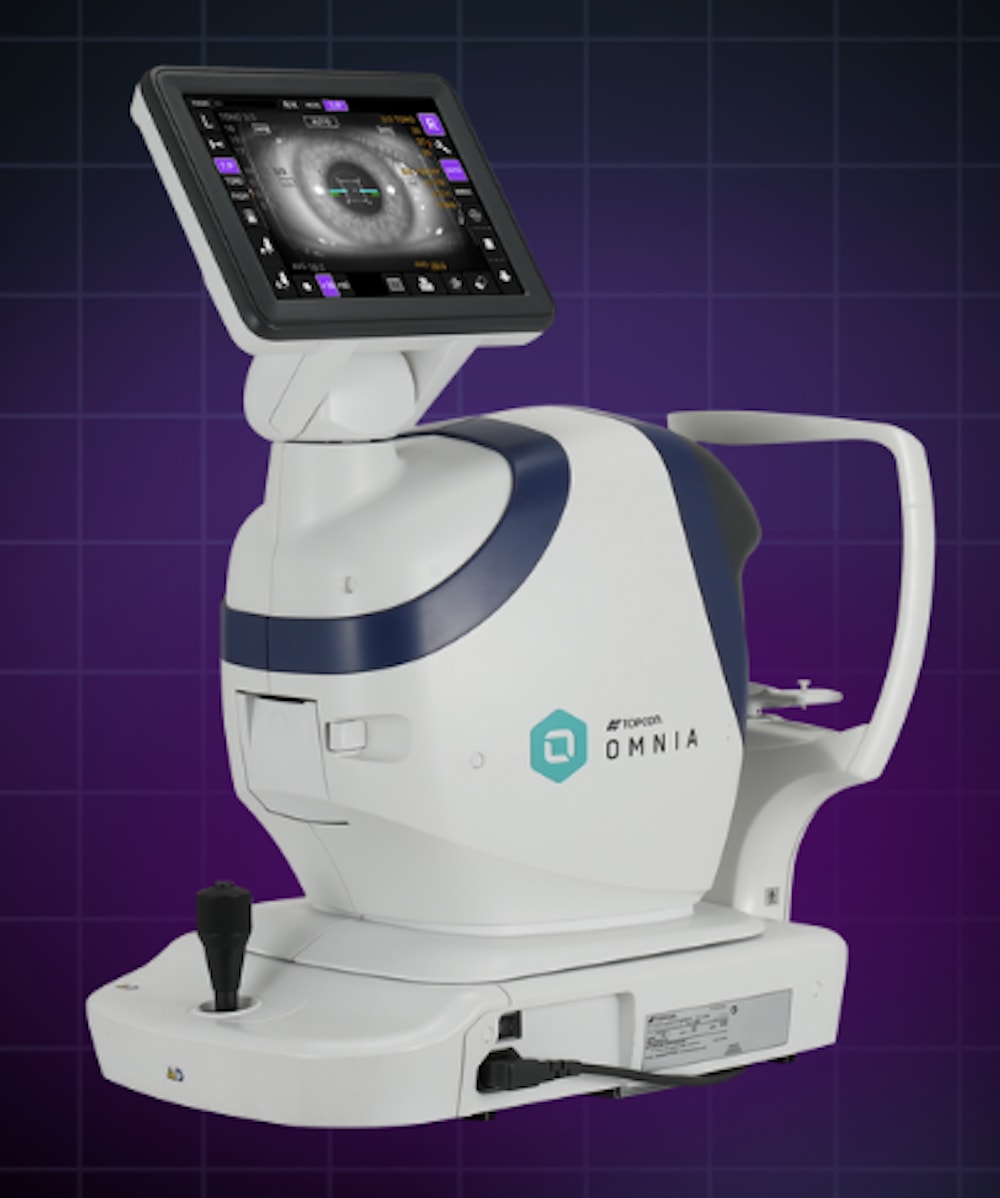
Topcon Healthcare, Inc. announced the FDA 510(k) clearance and commercial launch of Omnia, a fully automated 4-in-1 pretesting solution that integrates objective refraction, keratometry, tonometry, and pachymetry into a single device. Topcon said in a press release that Omnia is designed to enhance efficiency across diverse practice settings—enhancing workflow, shortening training time, and providing reliable data even when operated by new users.
According to Topcon, by consolidating objective refraction, keratometry, tonometry, and pachymetry into a single unit, the Omnia saves space and improves the speed and efficiency of the pretest workflow. The 360° rotating screen also supports space optimization and efficiency by enabling flexibility in patient positioning and device placement. DICOM compatibility allows for integration into digital workflows for efficient data sharing with electronic medical records.
In addition, the company said Omnia combines several features to ensure reliable measurements and patient comfort. Topcon’s Advanced Rotary Prism Technology is designed to reduce artifact from the fundus. The super luminescent diode delivers brighter, higher-quality imaging than LED, ensuring reliable measurements even in challenging conditions caused by media opacities such as cataracts. Also, the device’s quiet and softer air puff enhances patient comfort, the company noted.
In addition, Topcon said, Omnia’s automatic alignment function ensures accurate readings at the push of a button, while the optional manual mode allows for precise adjustments in complex cases. Practitioners can program device settings for their specific preferences, selecting the tests—(objective refraction, keratometry, tonometry, or pachymetry) that support their clinical approach.