Ligneous conjunctivitis is the most common clinical manifestation of plasminogen deficiency type 1 (PLGD-1), with 81% of patients presenting with ocular involvement. PLGD-1 is a systemic protein deficiency characterized by the formation of ligneous lesions on mucosal membranes throughout the body. The lesions are often inflamed and painful and, if left untreated, can lead to organ dysfunction and failure, including vision impairment and blindness.1 With the FDA approval of RYPLAZIM (plasminogen, human-tvmh; Kedrion Biopharma, Inc.), there is now an effective treatment for PLGD-1.2
Here, we follow the journey of a patient with PLGD-1, along with general insights on this disorder from Angela Y. Zhu, MD.
Manifestation
A 14-year-old female patient developed manifestations of plasminogen deficiency type 1 (PLGD-1) as an infant. At 11 months, an otolaryngologist noted a “fibrotic” tympanic membrane and performed a tympanostomy tube placement.
At age 3, the patient presented with conjunctival lesions initially diagnosed as bacterial conjunctivitis. Despite topical and oral antibiotics, the lesions persisted, and she was then referred to an ophthalmologist, who performed weekly debridement of the pseudomembranes. At age 4, she underwent her first surgical excision of the conjunctival lesions, which began to recur within 4 hours.
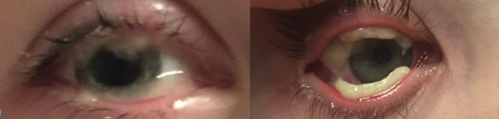
Diagnosis
At age 5, a second surgical excision revealed a histopathological diagnosis of fibrous membranes consistent with ligneous conjunctivitis. Laboratory testing confirmed reduced plasminogen activity, establishing the diagnosis of PLGD-1.
Treatment and Outcomes
When the patient began to lose vision, a third surgery was performed for conjunctival lesion removal. After developing urinary and reproductive tract lesions, the patient was enrolled in the RYPLAZIM clinical trial and began regular infusions. According to her mother, visible improvement occurred within hours of the first infusion. During a break in treatment due to drug shortage, the patient developed gingival lesions, which resolved when the infusions restarted.
Now in her eighth year of regular treatment, the patient remains free of recurrent symptoms and leads an active life, including participation in cheerleading, swimming, and dancing.
Insights into PLGD-1
By Angela Y. Zhu, MD
This patient’s course highlights several diagnostic and therapeutic challenges in the management of PLGD-1.
Ligneous (woody) pseudomembranous or membranous lesions on the conjunctiva and other mucous membranes are often the earliest and most visible manifestations, yet PLGD-1 is systemic and can affect multiple organ systems, with over 80% of patients demonstrating multi-organ involvement.1 Persistent or recurrent lesions that are unresponsive to standard therapies should prompt consideration of PLGD-1, with confirmatory testing via serum plasminogen activity levels. Untreated PLGD-1 can lead to serious complications, including vision loss, hearing loss, infertility, renal and respiratory failure, and destruction of teeth and surrounding bone.3
RYPLAZIM, the first FDA-approved therapy for PLGD-1, has demonstrated robust safety and efficacy: 78% of external and 75% of internal lesions resolved completely by week 48 of the pivotal phase 2/3 clinical trial. Additionally, improvement was observed in all remaining lesions. After 5 years of follow-up, there were no new or recurrent lesions.4
References
- Shapiro AD, Menegatti M, Palla R, et al. An international registry of patients with plasminogen deficiency (HISTORY). Haematologica. 2020;105(3):554-561. doi:10.3324/haematol.2019.241158.
- U.S. Food and Drug Administration. FDA approves first treatment for patients with plasminogen deficiency, a rare genetic disorder. Press release. Silver Spring, MD; June 4, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-plasminogen-deficiency-rare-genetic-disorder. Accessed September 2, 2025.
- Schuster V, Menegatti M, Palla R, et al. An international registry of patients with plasminogen deficiency (HISTORY). Haematologica. 2020;105(3):554-561. doi:10.3324/haematol.2019.241158.
- Shapiro AD, Nakar C, Parker JM, Thibaudeau K, Crea R, Sandset PM. Plasminogen, human-tvmh for the treatment of children and adults with plasminogen deficiency type 1. Haemophilia. 2023;29(6):1450-1458. doi:10.1111/hae.14849.
This editorial content is supported by Kedrion
INDICATIONS AND USAGE RYPLAZIM® (plasminogen, human-tvmh) is a plasma-derived human plasminogen indicated for the treatment of patients with plasminogen deficiency type 1 (hypoplasminogenemia). IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS: RYPLAZIM is contraindicated in patients with known hypersensitivity to plasminogen or other components of RYPLAZIM. WARNINGS AND PRECAUTIONS: Bleeding: RYPLAZIM administration may lead to bleeding at active mucosal disease-related lesion sites or worsen active bleeding not related to disease lesions. Discontinue RYPLAZIM if serious bleeding occurs. Monitor patients during and for 4 hours after infusion when administering RYPLAZIM to patients with bleeding diatheses and patients taking anticoagulants, antiplatelet drugs, or other agents which may interfere with normal coagulation. Tissue Sloughing: Respiratory distress due to tissue sloughing may occur in patients with mucosal lesions in the tracheobronchial tree following RYPLAZIM administration. Please monitor appropriately. Transmission of Infectious Agents: RYPLAZIM is made from human plasma and therefore carries a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob Disease (CJD) agent. Hypersensitivity Reactions: Hypersensitivity reactions, including anaphylaxis, may occur with RYPLAZIM. If symptoms occur, discontinue RYPLAZIM and administer appropriate treatment. Neutralizing Antibodies: Neutralizing antibodies (inhibitors) may develop, although they were not observed in clinical trials. If clinical efficacy is not maintained (e.g., development of new or recurrent lesions), determine plasminogen activity trough levels in plasma.Laboratory Abnormalities: Patients receiving RYPLAZIM may have elevated blood levels of D-dimer. D-dimer levels will lack interpretability in patients being screened for venous thromboembolism (VTE). ADVERSE REACTIONS: The most frequent (incidence ≥ 10%) adverse reactions in clinical trials were abdominal pain, bloating, nausea, fatigue, extremity pain, hemorrhage, constipation, dry mouth, headache, dizziness, arthralgia, and back pain. To report SUSPECTED ADVERSE REACTIONS, contact KEDRION at 1-855-427-6378 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
©️ 2025 Kedrion Biopharma Inc. All rights reserved. RYPLAZIM and the RYPLAZIM logo are registered trademarks of Kedrion Biopharma Inc.
RY-0263-00-2025









