Presentation
JT is an 86-year-old retired radar engineer who, prior to retirement, worked on radar components for the F-14 fighter jets. He was recently seen in my practice after being referred for evaluation of worsening macular degeneration. His optometrist has been following his dry macular degeneration for years and noticed his condition was progressing. The patient reports worsening ability to read without very bright light, particularly with scanning the lines going across the page. He reports no difficulty with his distance vision.
His past ocular history is significant for cataract surgery with intraocular implants in both eyes, and he is not currently using eye drops. He has no significant past medical history and is amazingly on no systemic medication.
Diagnosis
A comprehensive ocular examination including slit-lamp and dilated fundus examinations along with multimodal imaging including optical coherence tomography (OCT) and fundus autofluorescence (Figures 1 and 2) was performed. Snellen visual acuity was 20/25 in the right eye and 20/25 in the left eye. Slit-lamp examination was significant for posterior chamber intraocular lenses. Dilated fundus examination revealed bilateral nearly symmetric areas of geographic atrophy sparing the central fovea. There was no evidence of choroidal neovascularization.
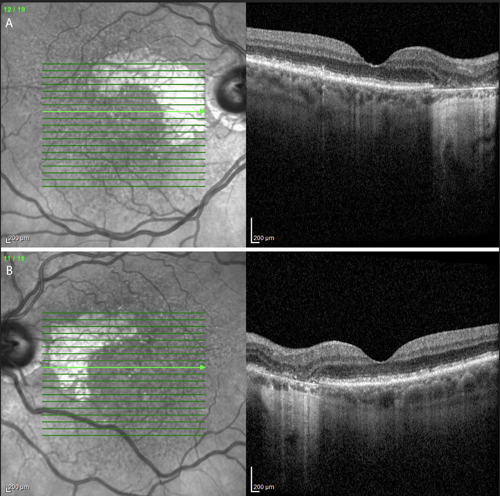
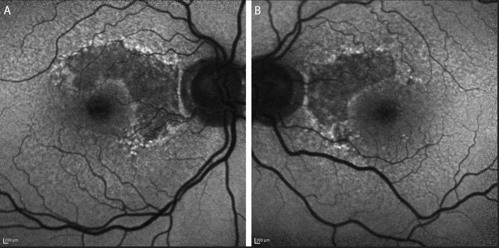
OCT highlighted the areas of retinal pigmentary atrophy along with focal loss of outer retinal morphology. Fundus autofluorescence revealed unifocal lesions of hypoautofluorescence with hyperautofluorescent edges, an imaging finding predictive of progression, in both eyes. The patient was diagnosed with age-related macular degeneration (AMD) with nonsubfoveal geographic atrophy (GA).
Treatment
Following the diagnosis of bilateral GA secondary to AMD, we discussed treatment options including FDA-approved therapies as well as the option of continued observation with AREDS vitamins and amsler grid at-home monitoring. We reviewed the risks and benefits of treatment along with the risks and benefits of observation. Following this discussion, the patient opted to proceed with treatment with an FDA-approved complement inhibitor. We discussed the on-label flexibility of treating monthly or every other month, and he opted for treatment every other month starting with the right eye followed by the left eye 4 to 5 weeks later.
Discussion
This patient is an ideal candidate for complement inhibition for his GA for several reasons. First, he has documented progression of his AMD disease over the years with his primary eye doctor. In addition, he has progressive visual symptoms consistent with his disease and his anatomic changes. Lastly, he has preservation of central vision due to his disease process remaining outside of his central fovea, so slowing down the growth of the GA with this treatment may preserve his central vision longer than would be achieved by observation alone.
Having an FDA-approved option for slowing the growth of GA secondary to AMD has revolutionized how these patients are managed. Patients must be counseled on the risks and benefits of treatment and must understand these therapies don’t reverse their GA. The GA on average will continue to grow, although at a slower rate.1 I do believe selecting the ideal candidates for these therapies will allow the retinal specialist to optimize their outcomes in treating patients with GA.
References
- Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet. 2023;402(10411):1434-1448. doi:10.1016/S0140-6736(23)01520-9









