Presentation
A 70-year-old male patient was referred to California Retina Consultants for evaluation of geographic atrophy (GA). He was effectively monocular: Vision in his left eye was already poor (VA 20/800), making his right eye (VA 20/40 cc) critical for his daily functioning. He reported increasing difficulty with reading and tracking words while reading, as well as problems adapting between light and dark environments. These functional changes heightened his fear of losing central vision in his better-functioning eye.
Diagnosis
On clinical examination and multimodal imaging—including optical coherence tomography (OCT) and fundus autofluorescence (Figures 1 and 2A)—we confirmed a GA lesion with hyperfluorescent edges, a hallmark of progression, in the right eye. Comparing current imaging of the patient’s right eye with earlier records demonstrated clear lesion growth (Figure 2). These prognostic factors, combined with his functional symptoms, made the patient an excellent candidate for therapy with an FDA-approved complement inhibitor. Prior to the availability of complement inhibitor therapy for GA, management of this condition was limited to monitoring every 6 months, with no effective intervention beyond lifestyle measures.
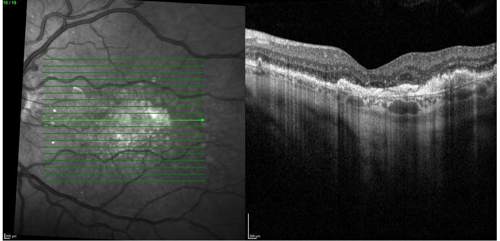
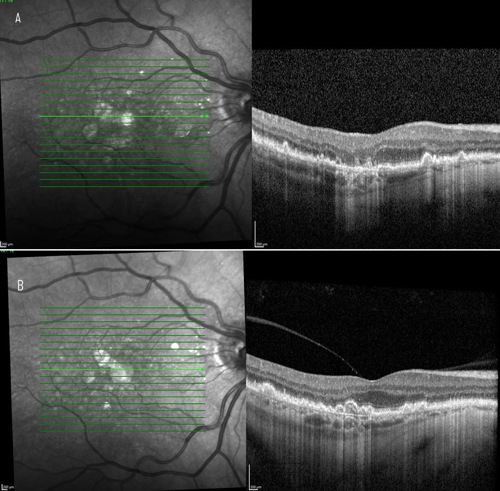
Treatment
Given the patient’s reliance on his better-functioning eye, I began treatment cautiously. Following my standard approach, I initiated an injection in the left eye to rule out inflammation or vasculitis before treating the better-functioning eye. Once safety was established, I proceeded with injections in the better-functioning right eye.
One important advantage of this therapy is its flexible dosing. It is FDA-approved for monthly or every-other-month administration. I typically prefer every-other-month dosing to reduce treatment burden and minimize the dose-dependent risk of side effects, such as intraocular inflammation or conversion to wet age-related macular degeneration (AMD). Clinical trial data suggest that efficacy is maintained with every-other-month dosing and the safety profile is improved compared with monthly use.
Outcome and Discussion
Unlike therapies for wet AMD, complement inhibitors for GA do not restore lost vision. Instead, they slow the progression of atrophy and help to preserve vision longer. In this patient’s case, the goal was to delay foveal involvement and maintain quality of vision for as long as possible. Based on trial data, treatment slows lesion growth by approximately 20% to 30% over a period of 2 to 3 years. While our current imaging tools cannot quantify his individual rate of slowdown of GA progression, evidence from large phase 3 trials (OAKS and DERBY, with long-term data from GALE)1,2 supports the likelihood that he is benefiting from treatment with a complement inhibitor.
Key Takeaways
This case underscores several key points for managing GA:
- Urgency matters. Beginning treatment before lesions encroach on the fovea is critical. Slowing progression before there is foveal involvement is far more impactful in preserving central vision than trying to slow progression once the fovea is already affected.
- Lesion location guides urgency. Non-subfoveal lesions, like those seen in this patient, are particularly important to treat early to preserve vision before foveal involvement.
- Flexible dosing is a differentiator. The ability to choose monthly or every-other-month dosing provides physicians with options to balance efficacy, safety, and patient burden.
- Long-term efficacy offers hope. Slowing GA progression preserves functional vision longer—a benefit that patients with GA value immensely.
For patients such as this one, the availability of complement inhibitor therapy has transformed GA management from watchful waiting to proactive treatment. While these therapies do not reverse disease, they represent a meaningful step toward preserving independence and quality of life for patients at risk of vision loss.
References
- Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet. 2023;402(10411):1434-1448. doi:10.1016/S0140-6736(23)01520-9
- Wykoff CC, Holz FG, Chiang A, et al; OAKS, DERBY, and GALE Investigators. Pegcetacoplan treatment for geographic atrophy in age-related macular degeneration over 36 months: data from OAKS, DERBY, and GALE. Am J Ophthalmol. 2025;276:350-364. doi:10.1016/j.ajo.2025.04.016
This editorially independent content is supported by 









