Ocular gene therapy offers potential treatments for previously incurable inherited retinal diseases (IRDs). Since the landmark FDA approval of voretigene neparvovec-rzyl (Luxturna; Spark Therapeutics, Inc.) in 2017 for RPE65-associated retinal dystrophy, the field has expanded dramatically, with numerous clinical trials exploring novel approaches.
KEY TAKEAWAYS:
• Gene therapy offers new treatment options for previously incurable inherited retinal diseases (IRDs).
• Optogenetics converts retinal ganglion cells or bipolar cells into artificial photoreceptors using opsins (light-sensitive proteins).
• Optogenetics is gaining momentum with multiple phase 2 trials, regulatory milestones, and the potential to become a transformative treatment for blindness due to IRDs.
Luxturna remains the pioneer in ocular gene therapy, providing treatment for patients with confirmed biallelic RPE65 mutations causing inherited retinal disease. This subretinal injection delivers a functional copy of the RPE65 gene using an adeno-associated virus (AAV) vector.1 Long-term follow-up studies have shown both successes and challenges. While patients experienced significant functional improvements, some developed progressive post-treatment pigmentary changes.
In March, revakinagene taroretcellwey (Encelto; Neurotech Pharmaceuticals, Inc.), became the first FDA-approved treatment for idiopathic macular telangiectasia (MacTel) type 2.
Unlike traditional gene replacement therapies, Encelto uses an encapsulated cell therapy with retinal pigment epithelial cells genetically engineered to continuously secrete ciliary neurotrophic factor (CNTF). Delivered as an intravitreal implant, Encelto slows photoreceptor degeneration, with clinical trials demonstrating significantly reduced progression of ellipsoid zone loss compared to sham procedures over 24 months. The innovative semi-permeable membrane design protects the implanted cells from the immune system while allowing CNTF to diffuse into the vitreous and retina, providing sustained therapeutic delivery.2
Luxturna and Encelto exemplify 2 distinct therapeutic strategies: targeted gene replacement for specific mutations and continuous delivery of neurotrophic factors to preserve retinal cells. However, another promising gene-agnostic category called optogenetics represents a different approach to treating retinal degeneration, particularly for patients with advanced disease where few or no photoreceptors remain.
Enter Optogenetics
Optogenetics addresses a fundamental challenge in retinitis pigmentosa (RP) and other IRDs: By the time most patients are diagnosed, significant photoreceptor loss has already occurred, making gene replacement therapies that target photoreceptors less effective. Optogenetic therapies aim to restore visual function by expressing artificial photoreceptors within surviving retinal neurons like bipolar cells or retinal ganglion cells (RGCs) [Figure 1]. In a landmark 2021 study, Sahel and others achieved the first partial restoration of visual function in a blind patient using optogenetic therapy with ChrimsonR and specialized goggles.3
Several companies are sponsoring clinical development of optogenetic therapies, each with distinct approaches and technologies. The following is an overview of each.
AbbVie/RetroSense Therapeutics
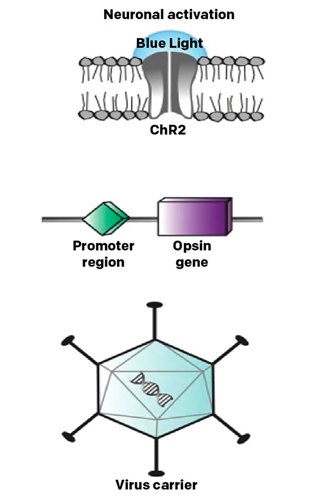
The RST-001 trial (NCT02556736) was one of the earliest optogenetic studies. It used channelrhodopsin-2 (ChR2) delivered via AAV [Figure 2] to make retinal ganglion cells light-sensitive in patients with advanced retinitis pigmentosa. This phase 1/2 trial has been completed, though detailed findings have not been widely published in peer-reviewed literature.
This trial helped establish the safety profile of optogenetic approaches and paved the way for later innovations. The original ChR2 opsin used in this study has lower light sensitivity and is activated by blue light, which later studies have tried to improve upon with red-shifted variants to reduce phototoxicity concerns.
GenSight Biologics
GenSight Biologics’ GS030 combines an AAV2.7m8 vector encoding the red-shifted channelrhodopsin ChrimsonR with light-stimulating goggles (GS030-MD). This approach targets retinal ganglion cells and uses special goggles to amplify light to appropriate wavelengths and intensities.
The PIONEER trial is a phase 1/2 dose-escalation study (NCT03326336) evaluating this combination in patients with non-syndromic retinitis pigmentosa. According to a January 2025 GenSight shareholder newsletter, “The follow-up of GS030 patients (our optogenetic product candidate for retinitis pigmentosa), treated as part of the phase I/II PIONEER clinical trial, is ongoing. Interim study results are expected over the course of 2025.” 4
Technology Drives Progress
Several technological innovations contribute to the rapid advancement of optogenetics.
Enhanced Opsins
Many clinical programs are using opsins, each with distinct properties:
ChR2 (AbbVie’s RST-001): The original channelrhodopsin-2 used in early studies, which has lower light sensitivity and is activated by blue light.
ChrimsonR (GenSight’s GS030): This red-shifted channelrhodopsin responds to amber light rather than blue, reducing phototoxicity risks while providing sufficient light sensitivity when paired with augmenting goggles.
MCOs (Nanoscope): These engineered multicharacteristic opsins aim to function across a broad spectrum of visible light and at ambient light levels, potentially eliminating the need for specialized goggles.
Chronos (Bionic Sight’s BS01): This opsin was chosen for its greater light sensitivity and temporal precision compared to original channelrhodopsins, potentially allowing for better visual information processing.
Researchers are closely watching how different opsins perform in the clinic, particularly whether faster response times, spectral sensitivity, or enhanced light sensitivity might translate to better patient outcomes without compromising safety.
Strategic Target Cell Selection
Different optogenetic approaches target different retinal cells, with potential implications for the quality of restored vision.
Retinal ganglion cells (RGCs) AbbVie, GenSight, and Bionic Sight target these cells. This approach is effective for advanced disease because RGCs often remain viable even after complete photoreceptor loss. However, targeting RGCs bypasses the natural processing of the retina’s middle layers.
ON-bipolar cells. Nanoscope targets these. Bipolar cells are more proximal to the lost photoreceptors in the visual pathway, potentially preserving more of the retina’s natural processing capabilities.
The optimal cell target may depend on the patient’s disease stage, with earlier-stage patients potentially benefiting more from bipolar cell targeting. Those with very advanced degeneration may only have RGCs remaining as viable targets.
Complementary Technologies
The following technologies enhance optogenetic vision restoration:
Light-amplifying goggles, such as GenSight’s GS030-MD, address the challenge of insufficient light sensitivity by detecting visual information and projecting amplified light pulses at specific wavelengths onto the retina.
Neural coding devices like those used by Bionic Sight aim to translate visual information into patterns of light stimulation that mimic natural retinal code, potentially delivering more meaningful visual information to the brain
—LMC & TAC
Nanoscope Therapeutics
Nanoscope employs ambient-light-activating opsin technology that does not require specialized goggles or external devices. Their MCO technology is designed to be sensitive to a broad spectrum of wavelengths within
visible light.
Nanoscope has sponsored clinical trials targeting retinitis pigmentosa (RP) and Stargardt disease. Their vMCO-I trial (NCT04919473) was a phase 1/2 dose-escalation study evaluating the safety and tolerability of their intravitreal multi-characteristic opsin in patients with RP. The RESTORE trial (NCT04945772) is a completed phase 2 study that evaluated their Multi-Characteristic Opsin (MCO-010) therapy in adults with retinitis pigmentosa. This randomized, triple-masked trial compared the gene therapy to sham injections. Nanoscope announced that 88.9% (16/18) of treated patients experienced clinically meaningful improvements in vision-guided mobility or object recognition, and 94.4% (17/18) experienced clinically meaningful improvement in vision-guided mobility or visual acuity.5
The STARLIGHT trial is a completed phase 2 study (NCT05417126) that extended Nanoscope’s optogenetic therapy (vMCO-010) to patients with Stargardt disease. Results showed that patients with predominantly macular atrophy experienced clinically meaningful improvements in visual acuity, with MCO-010 patients exhibiting approximately a 3 dB gain in mean sensitivity.5
The FDA has granted MCO-010 Orphan Drug and Fast Track designation for both RP and Stargardt disease. Nanoscope intends to file a BLA submission in H1 2025.6
Bionic Sight
Bionic Sight’s approach combines optogenetics with a neural coding device that translates visual information into patterns that mimic the retina’s natural code. The company’s BS01 trial (NCT04278131) is a phase 1/2 study evaluating their optogenetic therapy (BS01), developed to treat RP patients with advanced-stage vision loss.
Patients who received the lower doses showed a modest but consistent improvement: 5 of 5 showed an increase in light sensitivity of 100-fold or more, and several also gained the ability to detect motion. Patients who received the 2 highest doses showed more marked improvement. Ten of 11 showed an increase in visual acuity, with 7 patients showing an increase on the EDTRS eye chart averaging nearly 5 lines of improvement.7
In February, Bionic Sight received Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA for BS01. The company is now preparing its phase 3 program as it advances toward marketing approval.7
Ray Therapeutics
Ray Therapeutics’ RTx-015 uses a rhodopsin-based approach that may show increased light sensitivity compared to channelrhodopsins. The company recently initiated the ENVISION trial (NCT06460844), a phase 1 study exploring the use of RTx-015 in patients with either RP or choroideremia, and isdeveloping a second program, RTx-021, which targets retinal bipolar cells.8
Zhongmou Therapeutics
Zhongmou Therapeutics is conducting the MOON trial (NCT06292650), an early phase 1 study testing multiple doses of ZM-02 in patients with RP. In November 2024, the company announced that the USFDA granted Orphan Drug Designation (ODD) for ZM-02 optogenetic therapy to treat patients with RP without specific genetic restrictions.9
Challenges to Overcome
Despite the promising progress, several challenges remain.
Light sensitivity limitations: Even with improved opsins, most optogenetic therapies still require higher light levels than normal vision, necessitating complementary technologies or further opsin engineering.
Visual quality: Current approaches can restore basic visual functions, but patients still lack detailed acuity and color discrimination.
Retinal remodeling: Advanced degeneration causes complex changes in retinal architecture that may limit optogenetic effectiveness.
Long-term safety and durability: While early results are encouraging, the long-term safety and durability of effect remain to be established.
Vision rehabilitation: Patients receiving optogenetic therapy needtraining to interpret new visual signals, which requires specialized rehabilitation protocols.
Conclusion
Optogenetics has made remarkable progress since its inception in 2004, with multiple clinical trials spanning different approaches to address inherited retinal disease in a gene-agnostic fashion. As this technology matures, optogenetics will expand treatment options for those affected by devastating inherited retinal degeneration. OM
References
1. Luxturna US prescribing information. https://www.gene.com/download/pdf/luxturna_prescribing.pdf
2. Encelto US prescribing information. www.neurotechpharmaceuticals.com/wp-content/uploads/ENCELTO- PRESCRIBING-INFORMATION.pdf
3. Sahel, JA, Boulanger-Scemama, E., Pagot, C et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat Med 27, 1223–1229 (2021). https://doi.org/10.1038/s41591-021-01351-4
4. GenSight Biologics Newsletter to shareholders January 2025. www.gensight-biologics.com/wp-content/uploads/2025/01/Gensight_News- letter_20250127_EN.pdf
5. Nanoscope Therapeutics clinical trials. www.nanostherapeutics.com/pipeline/clinical-trials/
6. Nanoscope Therapeutics pipeline. www.nanostherapeutics.com/pipeline/
7. Bionic Sight’s BS01 Gene Therapy Receives RMAT Designation from the FDA. News release. February 18, 2025. www.globenewswire.com/news-release/2025/02/18/3027640/0/en/Bionic-Sight-s-BS01-Gene-Therapy-
Receives-RMAT-Designtion-from-the- FDA.html8.
8. Study to Evaluate Safety of RTx-015 Injection in Retinitis Pigmentosa or Choroideremia Patients (ENVISION) Ray Therapeutics clinical trials. https://raytherapeutics.com/clinical- trials/
9. Zhongmou Therapeutics. Zhongmou Announces Groundbreaking Clinical Data for ZM-02 and ZM-01 at ARVO and ASGCT 2025, Recognized with ARVO Foundation Travel Grant. News release. April 18, 2025. www.zmtherapeutics.com/en/news/










