Dry eye disease (DED) is an exceedingly common condition, with a prevalence of up to 50% of the global population.1 DED can considerably impact patients’ quality of life and can also sour ophthalmologists’ otherwise positive clinical, surgical and refractive outcomes. Therefore, proper diagnosis and targeted management of DED is essential to the modern ophthalmologist.
The challenge, however, with DED diagnosis is that it is not one disease but an umbrella term for many etiologies, such as meibomian gland dysfunction (MGD), aqueous-deficient dry eye, allergic ocular surface disease and neurosensory abnormalities, among others.2
Moreover, there are many DED masqueraders, such as neurotrophic keratitis, limbal stem cell deficiency and superior limbic keratoconjunctivitis. Proper differentiation amongst these DED subtypes and masquerading conditions is essential to determining the appropriate, targeted treatment and to encourage patient compliance and “buy-in” to support our efforts in management.
Fortunately, we have many diagnostic tools for DED — several of which combine multiple testing modalities. However, it remains challenging to seamlessly integrate these into clinical practice. One fundamental challenge is striking a balance between under-investigation (which may not lead to a diagnosis) and over-testing (which may be physically uncomfortable for patients, not to mention taxing within the workflow of a modern ophthalmic practice).3
A systematic approach to dry eye testing not only yields the appropriate diagnoses, but also enhances clinic flow, allows opportunities for patient education and, ultimately, optimizes clinical outcomes. To aid in refining an approach to testing for DED, we will summarize some of the modern DED diagnostic tests and devices, consider how these may be integrated into routine clinical practice and review our version of the dry eye clinic appointment.
ADVANCED DRY EYE TESTING DEVICES
An array of devices are now available for clinical use. Broadly, these devices either measure tear osmolarity, conduct specialized testing of biomarkers within the tear film or carry out specialized imaging of the ocular surface. Though discussing every available device is beyond the scope of this article, a selection of modern dry eye testing tools is summarized in the Table, below.

Within the multifactorial etiology of DED, tear film hyperosmolarity is a key outcome of aberrant physiology and contributes considerably to the pain experienced by patients.4 Osmolarity values reported within the literature range from ~302 mOsm/L for normal, ~315 mOsm/L for mild-moderate DED and ~336 mOsm/L for severe disease.4 Multiple devices exist for osmolarity measurement and are described in more detail in the first section of the Table.
Direct sampling of biomarkers also holds diagnostic utility in DED. Measurement of specific proteins, such as matrix metalloproteinase-9 (MMP9) or immunoglobulin E (IgE), can provide insight into whether ocular surface inflammation or an allergic eye condition is present, and thus aid in delivering targeted therapies.5 Devices used to measure biomarkers from the tear film are outlined in the second part of the Table.
Finally, non-contact imaging of the ocular surface also holds considerable utility in the diagnosis of DED. Imaging devices allow for objective, reproducible assessment of key aspects of ocular health, including the tear film, meibomian glands and non-invasive tear break-up time
(NITBUT).6 Imaging devices and their utility is discussed in detail in the third part of the Table.
PUTTING IT TOGETHER — OUR DRY EYE APPOINTMENT
Reviewing these diagnostic devices highlights just how much technology is out there — and how difficult it can be to decide which tools to integrate into your practice. We like to follow a systematic approach to our dry eye clinic appointments, guided by evidence from validated algorithms in the literature, including the TFOS DEWS II Diagnostic Methodology Report, the ASCRS Algorithm for Preoperative Diagnosis and Management of Ocular Surface Disease,
the CEDARS-ASPENS Protocol, and the AAO’s Preferred Practice Pattern for Dry Eye Syndrome.7-10 Here’s our approach.
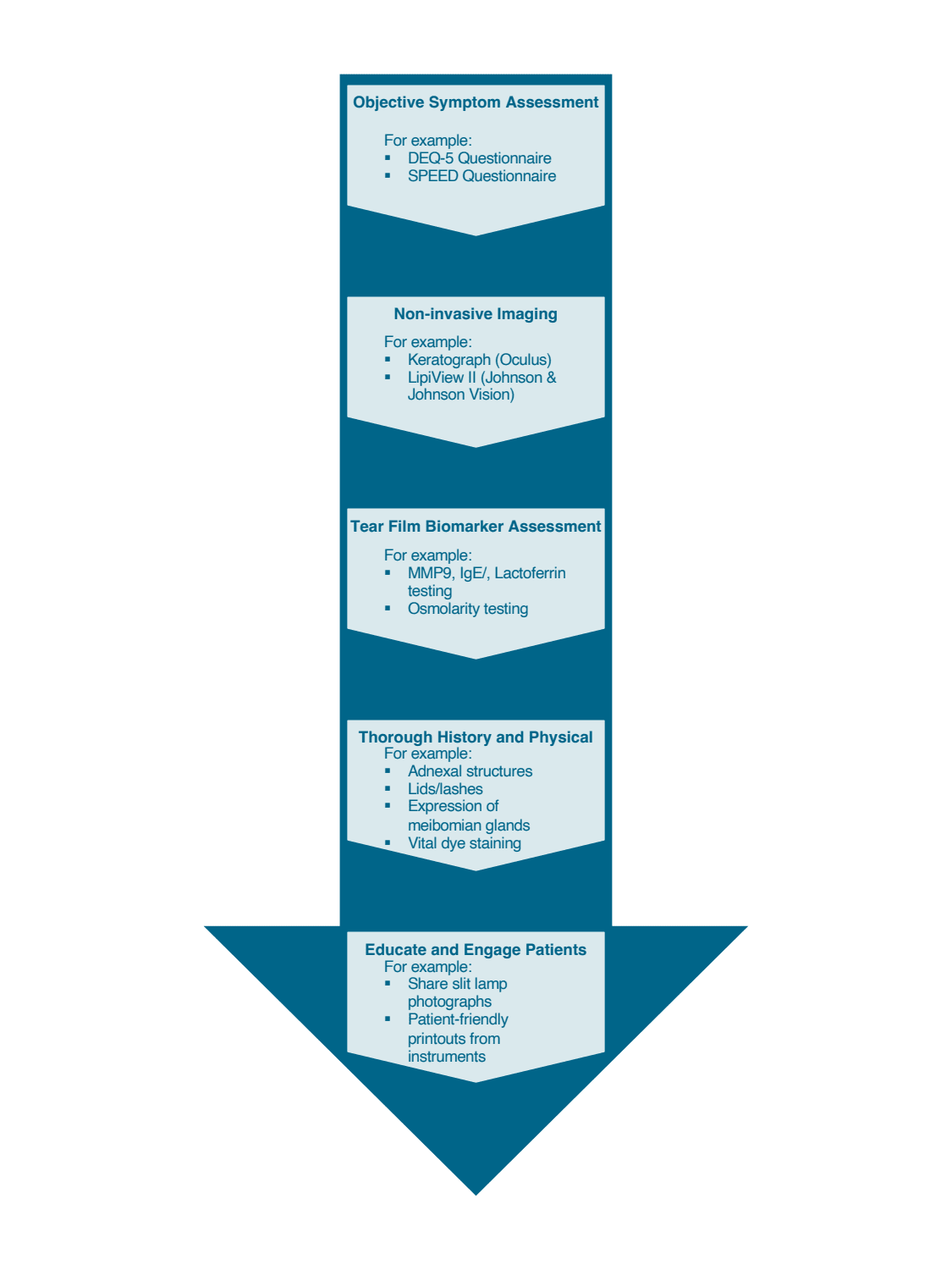
Begin with objective assessment of patient symptoms
The patient experience can be a tremendously helpful tool in the diagnosis of DED, and there exist several validated questionnaires to quantify symptoms. We like the Dry Eye Questionnaire 5 (DEQ-5) and the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaires, which are easy to administer and assess. These tests allow for objective tracking of patient symptoms over time and can aid in ensuring compliance to therapy and guide modifications, if necessary.

Utilize non-invasive imaging to assess the ocular surface
Once a questionnaire is completed, prior to instillation of any drops, patients are led to our imaging suite, where we use the Keratograph (Oculus) to obtain non-contact multi-modal imaging of the ocular surface. The CrystalTear Report generated by the Keratograph allows us to assess multiple aspects of the ocular surface as well as external anatomy, as shown in the sample report in Figure 1. We will sometimes substitute the Keratograph imaging with the LipiView II (Johnson & Johnson Vision) for the infrared meibography, as well as to obtain detailed assessment of the tear film’s lipid layer and further assess the blink rate (Figure 2). For patients who are presenting for pre-operative assessment for cataract or refractive surgery, we obtain and look closely at Placido-disc based topography to further characterize tear film integrity and stability.

Assess tear film quantity, quality and composition
Following imaging, we proceed with objective testing of the tear film. In our practice, we routinely conduct testing for MMP9 as well as measurement of tear osmolarity. In cases where we suspect an aqueous deficiency, we also proceed with Schirmer testing.11
Conduct a thorough history and clinical examination
Finally, once all testing is complete, our patients are brought to the consultation room, where we have already reviewed their testing and proceed with a detailed history, with inquiries guided by responses on the earlier questionnaires as well as reported symptoms. We then perform a complete physical examination, including assessment of the adnexal structures, keeping an eye out for signs of rosacea, eczema or lid malpositioning. Next, we proceed with a dynamic slit lamp examination, taking time to express the meibomian glands to evaluate secretions and function, and assess the ocular surface and TBUT with a staining test (typically fluorescein; Figure 3).

Involve patients in understanding their diagnosis
After completing a clinical evaluation, we like to review all testing with our patients. Many testing devices offer patient-friendly printouts, which can help in achieving understanding of their diagnosis and “buy-in” for targeted therapy. Patients are empowered to take charge of their care, understand their therapies and often will track their progress better than we ever can, optimizing their final outcomes.
PEARLS FOR BUILDING YOUR OWN DRY EYE WORKFLOW
Though our appointments make use of both non-contact imaging and point-of-care tear film testing technology, we do sometimes substitute these if unavailable, such as when we lack sufficient technical support that day or if the physician is working at a location that does not have access to the technology in question. If a Keratograph or LipiView II is not available, we default to a simple slit lamp photo with fluorescein staining, both to act as an objective measure for tracking disease and to aid in patient education. Possible drawbacks to this alternative include an inability to obtain infrared imaging of the meibomian glands directly, and lack of the objective readouts that are presented from the testing modalities; however, a slit lamp exam with expression of the meibomian glands and staining with fluorescein provides a significant amount of information that can be considered as a good substitute.
If MMP9 testing is not available, a reasonable alternative may be to consider osmolarity testing or another point-of-care biomarker test from the list described above.
Follow-up visits in our practice typically involve more focused, targeted testing rather than repeating the complete protocol. This not only streamlines clinic flow but makes it easier to track objective measures for each patient’s specific DED subtype, all the while maintaining patient “buy-in.” Our testing protocol remains dynamic, and we often modify which tests we conduct depending on the etiology of the disease — there is no “one-size-fits-all” approach when it comes to diagnosing DED. Challenging or refractory presentations typically require additional testing and modification of the protocol in accordance with the underlying etiology.
In building your own dry eye diagnostic workflow, consider a similar pipeline (see flowchart, below): begin with an objective questionnaire, include some form of imaging (multimodal if possible, though slit-lamp imaging is an acceptable substitute) and incorporate at least one objective tear film biomarker (osmolarity, MMP9 testing, etc). Such an approach should provide a balance between ensuring an appropriate diagnosis is reached while keeping clinic flow efficient and patients comfortable.
TAKEAWAYS
DED is a complex, multifactorial condition and accurate diagnosis can be challenging. There are a plethora of imaging and point-of-care testing options available for diagnosis and monitoring of DED. At the core of all our dry eye clinic appointments remains a thorough clinical history and dynamic slit lamp examination. While advanced testing modalities are helpful, they remain adjuncts to careful clinical evaluation and listening to the patient. Integration of a multimodal approach to DED testing with advanced devices may not only aid in diagnosis but also in achieving patient “buy-in” to obtain optimal clinical outcomes. OM
References
1. Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15:334-365.
2. Galor A, Felix ER, Feuer W, Levitt RC, Sarantopoulos CD. Corneal nerve pathway function in individuals with dry eye symptoms. Ophthalmology. 2021;128:619-621.
3. Uchino M, Yokoi N, Shimazaki J, Hori Y, Tsubota K, on behalf Of The Japan Dry Eye Society. Adherence to eye drops usage in dry eye patients and reasons for non-compliance: A web-based survey. J Clin Med Res [Internet]. 2022;11(2). Available from: http://dx.doi.org/10.3390/jcm11020367.
4. Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438-510.
5. Kumar NR, Praveen M, Narasimhan R, Khamar P, D’Souza S, Sinha-Roy A, et al. Tear biomarkers in dry eye disease: Progress in the last decade. Indian J Ophthalmol. 2023;71:1190-1202.
6. Han SB, Liu YC, Mohamed-Noriega K, Tong L, Mehta JS. Objective imaging diagnostics for dry eye disease. J Ophthalmol. 2020;2020:3509064.
7. Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15:539-574.
8. Milner MS, Beckman KA, Luchs JI, Allen QB, Awdeh RM, Berdahl J, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27 Suppl 1(Suppl 1):3-47.
9. Starr CE, Gupta PK, Farid M, Beckman KA, Chan CC, Yeu E, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45:669-684.
10. Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Lin A, Rhee MK, et al. Dry Eye Syndrome Preferred Practice Pattern. Ophthalmology. 2019;126:P286-334.
11. Holly FJ, Lamberts DW, Esquivel ED. Kinetics of capillary tear flow in the Schirmer strip. Curr Eye Res. 1982;2:57-70.










