Dry eye disease (DED) is a widespread condition with an estimated prevalence ranging from 5%-50% worldwide.1 While dry eye prompts many patients to seek out care, there remains a notable gap in meeting the need for effective dry eye treatments. The impact DED has on quality of life has been well-documented.2,3
The past few years, however, have shown a remarkable increase in the approval of dry eye therapeutics. As two clinicians who see numerous patients with DED and ocular surface disease (OSD), we are thrilled to now have a plethora of treatments to offer to our patients. In this article, we’ll discuss the most recently approved treatments.
Key Tips on When to Utilize These OSD Therapies
XDEMVY
Look at the lids! If collarettes are present, consider Xdemvy.
MIEBO
Evaluate the tear break up time — if rapid or less than 10 seconds, consider Miebo.
TYRVAYA
Consider Tyrvaya for contact lens wearers, those with difficulty applying drops or those with multiple drop burden.
XDEMVY
Xdemvy (lotilaner ophthalmic solution) 0.25% (Tarsus Pharmaceuticals) was approved in July 2023 for the treatment of Demodex blepharitis and has proven to be highly effective for our patients with stubborn Demodex infestations. Collarettes are a pathognomonic sign of Demodex and are easiest to see when the patient is looking down at the slit lamp. The waxy buildup of mite waste that forms a “collar” around the base of the lash is a telltale sign that Demodex are present. Demodex mites are extremely prevalent with studies showing that up to 58% of eye-care patients have Demodex.2,3
Common symptoms of Demodex blepharitis include dryness, burning and foreign body sensation and can mimic DED. Additionally, patients with Demodex also experience redness and itching along the lash line, crusting on lashes in the morning and can have a history of recurrent chalazia.
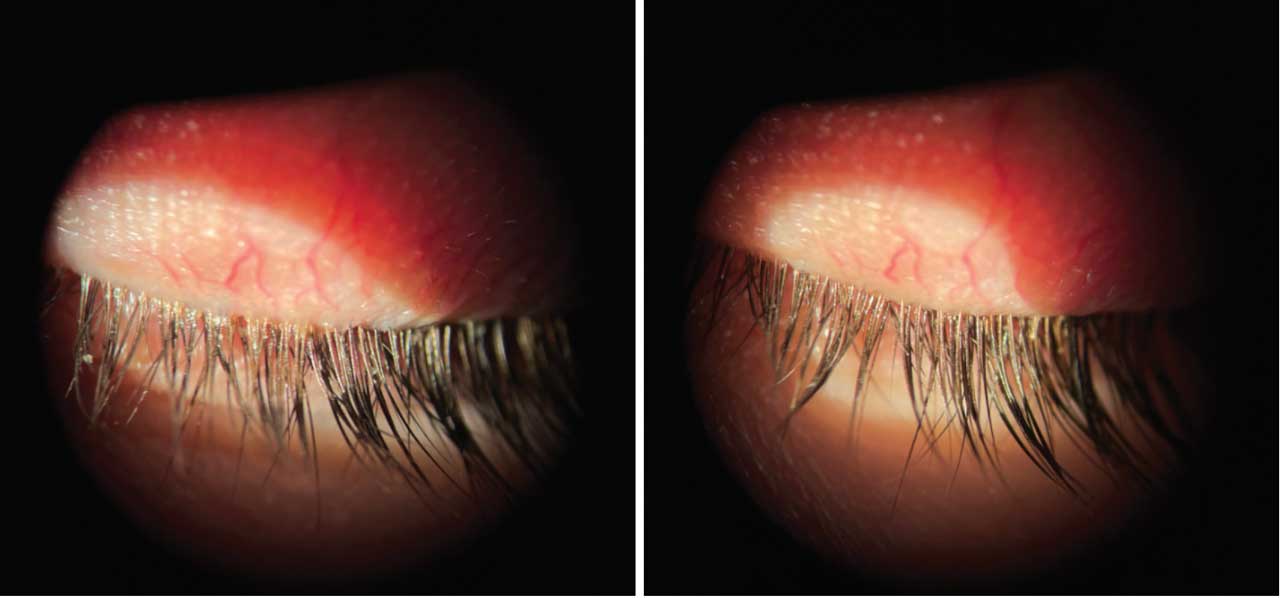
Various therapies such as in-office treatments and over-the-counter tea tree oil-based products have been utilized with varying success. Xdemvy is a great option for any patient with Demodex, but particularly for patients with OSD, meibomian gland dysfunction (MGD) and those who have tried other over-the-counter options without resolution.
Studies have shown that after the 6-week twice-a-day dosing, 85% of patients have less than 10 collarettes present, and 60% have zero collarettes.4 Because many other OSD treatments are used in perpetuity, our patients have been receptive to this treatment as it is only 6 weeks in duration and targets the root cause of Demodex blepharitis. In addition to Demodex eradication, one wonderful cosmetic improvement noted with Xdemvy is the improvement of eyelid erythema.
In the SATURN 1 and SATURN 2 clinical studies, lid erythema cure was seen in 25% of patients, which is particularly beneficial for patients who are cosmetically bothered by red-rimmed eyes.4 In a recently published observational study following patients for 1 year after the 6-week Xdemvy treatment, findings revealed 62% of patients treated with Xdemvy had 10 or less collarettes at day 365, indicating a lasting effect of the treatment.5
Achieving good clinical outcomes in our dry eye patients is a challenge when there is smoldering inflammation from a Demodex infestation on the lids. We are finding that starting patients on Xdemvy expediently can break the cycle of inflammation associated with Demodex mites.
Clinically, in addition to seeing eyelid collarette and erythema reduction, we are also observing improvements in associated blepharoconjunctivitis, marginal keratitis/corneal scarring and neovascularization and evaporative dry eye symptoms when addressing Demodex infestation using Xdemvy. Addressing Demodex in your patients will only enhance the efficacy of your existing dry eye treatments.
MIEBO
Miebo (Bausch + Lomb) has been an exciting adjunct to the dry eye armamentarium since its approval in May 2023.
Our understanding of the multifactorial nature of dry eye has grown in recent years, and we have identified evaporative dry eye as one of the predominant etiologies.6,7 When tear evaporation exceeds tear supply on the ocular surface, it can instigate a cycle of tissue desiccation and inflammation, causing patient discomfort and decreased vision. Increased rates of tear evaporation can occur in instances when patients have a reduced blink rate or incomplete blinks (ie, lagophthalmos, sustained screen time) or have conditions where the meibomian glands produce insufficient meibum despite a normal blink rate (ie, meibomian gland atrophy from medication use).
As such, the advent of Miebo has given clinicians a medication targeted specifically towards evaporative dry eye. Miebo is a preservative-free, steroid-free drop composed of 100% perfluorohexyloctane. While this medication does not target inflammation directly, it is a semi-fluorinated alkane that forms a monolayer at the air-tear interface and restricts tear evaporation. It is thought to mimic key functions of natural meibum and has a low surface tension that allows it to rapidly spread over the entire ocular surface. Clinical studies have shown statistically significant improvements in total corneal fluorescein staining and visual analog scale eye dryness scores from baseline line to 8 weeks of therapy with a low side effect profile. 8,9
Anecdotally, our patients have had a very positive response to Miebo with minimal side effects. The key is to identify patients who are experiencing evaporative dry eye. Evaluating for MGD, a rapid tear break-up time and subjective symptoms of fluctuating vision or feeling like drops don’t last long on the ocular surface can help identify these patients.
Dosed at four times a day, Miebo is relatively facile to integrate into a patient’s daily routine. The drop has worked well in patients who have been performing lid hygiene at home or who have had lid margin treatments (ie, thermal pulsation or intense pulsed light therapy) for MGD, with intermittent effect.
Post-refractive patients with evaporative dry eye have benefitted along with patients with meibomian gland damage from prior isotretinoin use. Miebo can also be used alongside topical immunomodulators in patients who have ocular surface inflammation as it is addressing DED via a different pathway. It can be utilized in conjunction with artificial tears use as well and as an alternative to tear formulations that have a lipid or
omega-3 component that are often used for evaporative dry eye.
TYRVAYA
Tyrvaya, or varenicline 0.03% (Viatris Inc.), has been another welcomed addition to the dry eye armamentarium since 2021. Compared with topical eyedrop therapies, this intranasal spray has been the first of its kind that is approved to treat the signs and symptoms of DED. The spray is preservative-free, water soluble and diffuses quickly across the nasal mucosa to bind with cholinergic receptors on the trigeminal nerve within the nasal cavity.
The trigeminal nerve provides the pathway for parasympathetic stimulation of the lacrimal functional unit and helps produce basal tears. By stimulating production of the patient’s own basal tears, patients can benefit from the therapeutic components of their endogenous tear film, as opposed to using various artificial tear formulations or topical anti-inflammatory medications.
Tyrvaya has played a large role in our practices. Patients who have used the medication enthusiastically have been contact lens wearers who appreciate the ease of using a nasal spray and not needing to remove their lenses when administering the spray and patients who have high eyedrop burden from other comorbidities (ie, glaucoma patients). We have also seen success in utilizing Tyrvaya for post thermal expression patients. Removal of gland obstruction prior to stimulating the lacrimal functioning unit appears to provide an additional benefit. Additionally, patients with arthritis who have trouble inserting drops can benefit from this unique drug delivery.
Dosed twice a day, onset of action has proven to be within a few weeks (often 2 weeks). Clinical trials showed 50% of patients had a ≥10-mm increase from baseline in Schirmer’s score at week 4.10,11
Patients are advised of side effects, including sneezing, coughing and throat irritation, that improve with consistent use. Educating patients that they do not need to inhale deeply with each spray keeps the medication in the nasal cavity and reduces the potential for throat irritation.
CONCLUSION
DED impacts all aspects of our practice from eyeglasses remakes to contact lens dropouts to poor surgical outcomes. Thankfully the landscape of dry eye treatment is experiencing a shift with the approval of exciting new treatments.
By targeting the underlying multifactorial mechanisms of DED such as Demodex and tear film instability, these therapies can significantly improve clinical outcomes and patients’ quality of life. OM
REFERENCES
1. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15:334-365.
2. Trattler W, Karpecki P, Rapoport Y. The prevalence of Demodex blepharitis in US eye care clinic patients as determined by collarettes: A pathognomonic sign. Clin Ophthalmol. 2022;16:1153-1164. Published 2022 Apr 15.
3. Rhee MK, Yeu E, Barnett M, et al. Demodex blepharitis: A comprehensive review of the disease, current management, and emerging therapies. Eye Contact Lens. 2023;49):311-318.
4. Yeu E, et al. Treatment of Demodex blepharitis with lotilaner ophthalmic solution, 0.25%: combined analysis of two pivotal, randomized, vehicle-controlled, multicenter trials. Saturn-1 and Saturn-2 combined data. Presented at: ARVO 2023; April 23-27, 2023; New Orleans.
5. Sadri E, Paauw JD, Ciolino JB, et al. Long-term outcomes of 6-Week treatment of lotilaner ophthalmic solution, 0.25%, for Demodex blepharitis: A noninterventional extension study. Cornea. Published online Feb. 9, 2024.
6. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring Study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799-806.
7. McCann P, Abraham AG, Mukhopadhyay A, et al. Prevalence and Incidence of Dry Eye and Meibomian Gland Dysfunction in the United States: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2022;140:1181-1192.
8. Tauber J, Berdy GJ, Wirta DL, Krösser S, Vittitow JL; GOBI Study Group. NOV03 for Dry Eye Disease Associated with Meibomian Gland Dysfunction: Results of the Randomized Phase 3 GOBI Study. Ophthalmology. 2023;130(5):516-524.
9. Sheppard JD, Kurata F, Epitropoulos AT, Krösser S, Vittitow JL; MOJAVE Study Group. NOV03 for Signs and Symptoms of Dry Eye Disease Associated With Meibomian Gland Dysfunction: The Randomized Phase 3 MOJAVE Study. Am J Ophthalmol. 2023;252:265-274.
10. Wirta D, Torkildsen GL, Boehmer B, et al. ONSET-1 Phase 2b randomized trial to evaluate the safety and efficacy of OC-01 (Varenicline Solution) nasal spray on signs and symptoms of dry eye disease. Cornea. 2022;41:1207-1216.
11. Wirta D, Vollmer P, Paauw J, et al. Efficacy and safety of OC-01 (Varenicline Solution) nasal spray on signs and symptoms of dry eye disease: The ONSET-2 Phase 3 Randomized Trial. Ophthalmology. 2022;129(4):379-387.










