Corneal opacities are responsible for at least 4.2 million cases of vision impairment globally.1 Since the world’s first successful penetrating keratoplasty (PK) was performed by Dr. Eduard Zirm in 1905, the landscape of corneal transplantation has greatly evolved, from the refinement of full-thickness corneal transplants to the introduction of lamellar keratoplasty procedures that allow for selective replacement of the diseased layers of the cornea.
Today, we are witnessing an exciting surgical revolution as the refinement and success of modern corneal transplantation provides decreased complication rates and improved clinical outcomes. The following review will bring you up to speed on the diverse corneal transplantation techniques available — highlighting their respective strengths and key postoperative complications — and the emerging advancements in the field.
PENETRATING KERATOPLASTY
PK involves complete replacement of the host cornea with a full-thickness donor corneal graft and can be utilized to treat a broad range of corneal disease. In PK, a trephine is used to make a full-thickness incision into the host cornea to remove the diseased tissue, which is then replaced with a donor corneal button that is sutured in place.
Although PK historically served as the gold standard of corneal transplantation, it is not without its challenges, particularly given the “open-sky” nature of the procedure and its increased risk of the dreaded complication of expulsive suprachoroidal hemorrhage. Primary graft failure, worsening glaucoma, microbial keratitis and wound dehiscence are among the most common postoperative complications.2
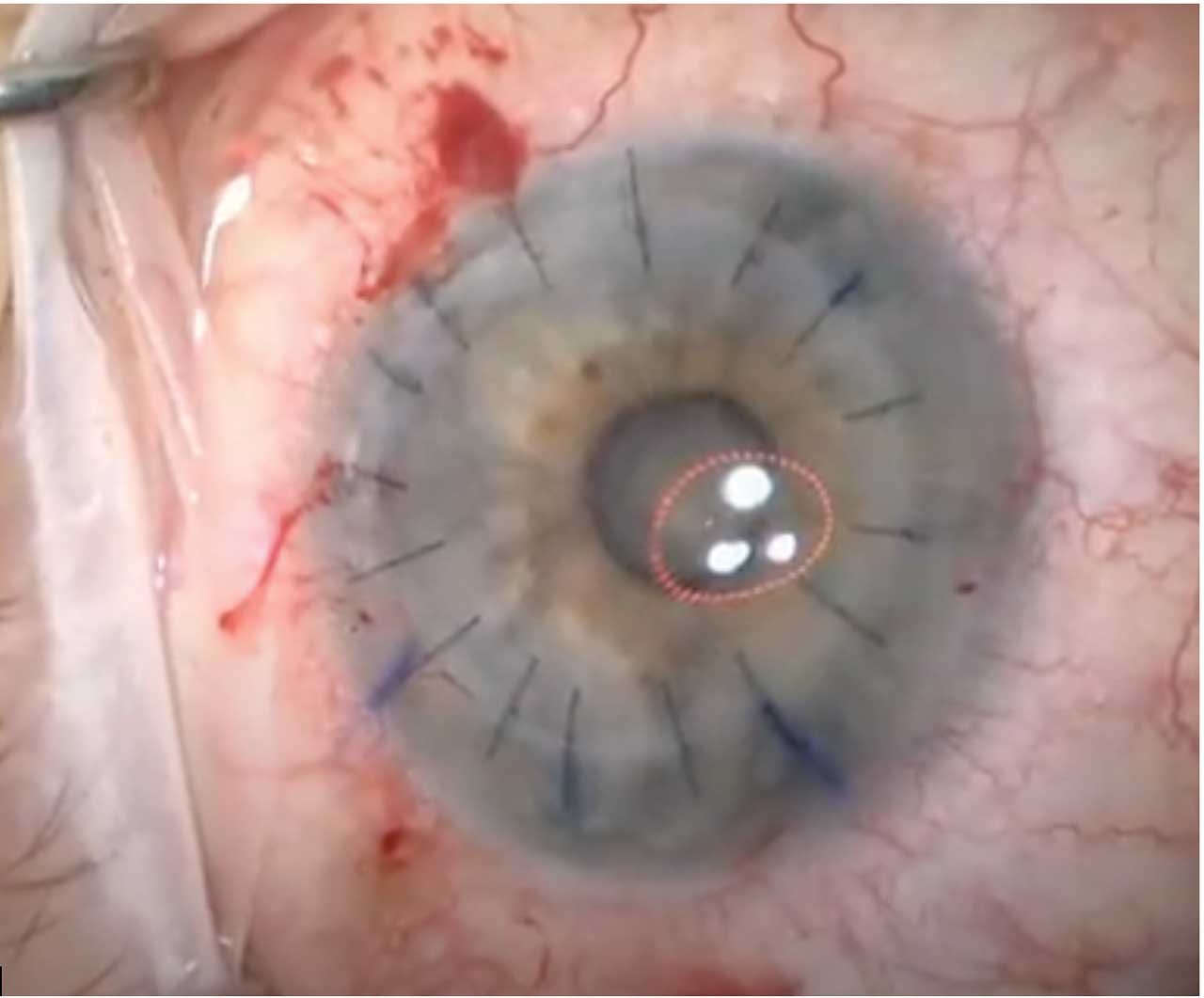
Induced astigmatism can also significantly affect visual outcomes following PK. Optimizing suture tension to achieve tissue apposition without inducing distortion and utilizing a keratoscope intraoperatively can help to mitigate postoperative astigmatism (Figure 1). The use of femtosecond laser technology has also been introduced as a means of creating better host-donor apposition in PK through more precise and customizable trephination options; however, clinical studies have not definitively shown a significant difference in long-term visual outcomes with the use of femtosecond laser compared to manual trephination.3
As the popularity of lamellar keratoplasty has increased in the past two decades, there has been a shift away from PK, but it remains the procedure of choice for combined stromal and endothelial pathologies.
ANTERIOR LAMELLAR KERATOPLASTY
To address the risks associated with full-thickness corneal transplantation, new techniques emerged that offered a more targeted and less invasive approach to corneal transplantation. Anterior lamellar keratoplasty (ALK), which was introduced in the early 1900s, consisted of a partial-thickness transplant of only donor epithelium and anterior stroma. Although interface irregularities initially limited visual outcomes and thus the popularity of the procedure, ALK experienced a revival with the introduction of the deep anterior lamellar keratoplasty (DALK) around 1984.
In DALK, a deep lamellar dissection is performed, followed by transplantation of anterior corneal donor tissue, leaving host Descemet membrane (DM) and endothelium intact. A technically difficult procedure, DALK was advanced by the introduction of the “big bubble” surgical technique,4 in which an air bubble is injected into the corneal stroma to cleave the posterior stroma from DM, allowing for a more uniform and smoother interface, with improved visual outcomes. Compared to PK, DALK has demonstrated lower rates of graft rejection and graft failure, in part due to the elimination of the risk of endothelial rejection given retention of the host endothelial layer, although the risk of epithelial and stromal rejection remains. Patients are also less likely to develop glaucoma complications after DALK, and, given the absence of a full-thickness incision, complications such as endophthalmitis and expulsive hemorrhage are reduced compared to PK.5
DALK remains a valuable technique for the treatment of stromal disease in the presence of healthy endothelium, such as in ectatic disorders, including keratoconus, or in the treatment of stromal scarring from infection or trauma.
ENDOTHELIAL KERATOPLASTY
At the end of the 20th century, Melles introduced the concept of posterior lamellar keratoplasty (PLK) to treat corneal endothelial disorders,6 laying the foundation for modern endothelial keratoplasty (EK). This exceptionally challenging surgical technique, later termed deep lamellar endothelial keratoplasty (DLEK), involves dissecting and excising the host posterior stroma, DM and endothelium while leaving the anterior corneal tissue intact. A donor lenticule replaces the posterior tissue and is inserted via a large sclerocorneal incision.
Melles later refined his technique to selectively remove only the host DM and endothelium, in a procedure called Descemet stripping EK (DSEK).7 The tissue removed is replaced with a manually dissected posterior lamellar donor button consisting of posterior stroma, DM and endothelium. An air bubble is then injected into the anterior chamber to encourage graft adherence. Additional advancements gave rise to Descemet stripping automated EK (DSAEK), in which lamellar dissection of the donor tissue is automated via the use of a microkeratome or femtosecond laser. Traditionally, donor grafts ranged from approximately 100-200 µm thick; however, recent studies suggest improved visual acuity and faster visual recovery with the use of thinner donor grafts, such as the ultra-thin DSAEK (60-90 µm) and nano-thin DSAEK (≤50 µm).
Compared to PK, DSAEK has demonstrated improved and faster visual rehabilitation, with minimal postoperative astigmatism, although visual acuity in DSAEK may still be limited by interface irregularities, uneven donor tissue dissection, and higher-order aberrations associated with posterior corneal curvature changes.8
Melles further revolutionized corneal transplantation surgery in 2006 with the introduction of DMEK,9 in which only DM and endothelium are transplanted after the descemetorhexis, with grafts as thin as 10-15µm. DMEK is a technically challenging procedure that requires difficult tissue preparation and careful manipulation of the delicate donor tissue intraoperatively.
Despite the technical difficulty of this procedure, DMEK surpassed PK as the most commonly performed corneal transplant procedure in the United States in 2012 and constituted almost 50% of total EK procedures in 2022.10 This was facilitated in large part by advancements in the tissue preparation performed by trained technicians in eye banks, including the availability of prestripped and preloaded DMEK tissue (Figure 2), which allows for surgeries to be performed more quickly and consistently.

DMEK offers improved and more rapid visual rehabilitation compared to DSAEK.11 DMEK has also demonstrated superior graft survival and lower rates of graft rejection compared to both DSAEK and PK.8 Generally, rejection and graft failure rates are reduced when the amount of transplanted tissue is also reduced.
Despite the low complication rate and excellent visual outcomes of DMEK, traditional DMEK methods require shallowing the anterior chamber intraoperatively and retaining a gas bubble postoperatively (Figure 3). For this reason, its utility may be limited in anatomically altered eyes, including unicameral eyes, post-vitrectomy eyes and eyes with glaucoma tubes shunts or large iridectomies. However, new techniques continue to emerge to allow for DMEK in eyes with altered anterior segment anatomy.

MORE INNOVATIONS TO COME
The landscape continues to evolve. With a Descemet stripping only, or DSO, procedure, a small descemetorhexis removes focal endothelial disease, allowing the remaining healthy endothelium to migrate across the bare posterior stroma. This does not require any donor tissue and, thus, does not carry a risk of rejection.
Cell therapy, synthetic prostheses and bioengineered grafts are also examples of innovative concepts currently under active investigation. In 2018, Kinoshito et al published a landmark study that demonstrated the efficacy of an injection of cultured human endothelial cells in restoring corneal function in patients with bullous keratopathy.12 In addition, various synthetic corneal tissue prostheses are currently being evaluated in clinical trials, including the CorNeat KPro (NCT05694247, CorNeat Vision), a synthetic cornea, and EndoArt (NCT05139771, EyeYon Medical), a synthetic endothelial prosthesis.
In a recently published Phase 1 study, Rafat et al implanted bioengineered porcine construct, double-crosslinked corneal tissue into an intrastromal pocket within the corneas of 20 advanced keratoconus patients. No adverse events were observed, and improvements in corneal thickness, maximum keratometry and visual acuity were documented.13 (For more on anticipated innovations, see “The future of corneal transplantation”.)
CONCLUSION
The last few decades have witnessed significant advancement in the field of corneal transplantation. These innovations have made technically complex corneal transplantation procedures more accessible and improved clinical outcomes for our patients. Continued research is warranted, of course. However, given the ongoing global shortage of donor corneal tissue,14 these encouraging results suggest a promising future. OM
References
- World report on vision. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO.
- Wagoner MD, Ba-Abbad R, Al-Mohaimeed M, Al-Swailem S, Zimmerman MB; King Khaled Eye Specialist Hospital Corneal Transplant Study Group. Postoperative complications after primary adult optical penetrating keratoplasty: prevalence and impact on graft survival. Cornea. 2009 May;28:385-394.
- Peng WY, Tang ZM, Lian XF, Zhou SY. Comparing the efficacy and safety of femtosecond laser-assisted vs conventional penetrating keratoplasty: a meta-analysis of comparative studies. Int Ophthalmol. 2021 Aug;41:2913-2923.
- Anwar M, Teichmann KD. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet’s membrane. Cornea. 2002 May;21:374-383.
- Arundhati A, Chew MC, Lim L, et al. Comparative study of long-term graft survival between penetrating keratoplasty and deep anterior lamellar keratoplasty. Am J Ophthalmol. 2021 Apr;224:207-216. Epub 2020 Nov 27.
- Melles GR, Eggink FA, Lander F, et al. A surgical technique for posterior lamellar keratoplasty. Cornea. 1998 Nov;17:618-626.
- Melles GR, Wijdh RH, Nieuwendaal CP. A technique to excise the Descemet membrane from a recipient cornea (descemetorhexis). Cornea. 2004;23:286-288.
- Woo JH, Ang M, Htoon HM, Tan D. Descemet Membrane Endothelial Keratoplasty Versus Descemet Stripping Automated Endothelial Keratoplasty and Penetrating Keratoplasty. Am J Ophthalmol. 2019 Nov;207:288-303. Epub 2019 Jun 19.
- Melles GR. Posterior lamellar keratoplasty: DLEK to DSEK to DMEK. Cornea. 2006;25(8):879-881.
- Mathews P, Benbow A, Corcoran K, et al. 2022 Eye banking statistical report—executive summary. Eye Banking and Corneal Transplantation. e0008-12, September 2023.
- Tourtas T, Laaser K, Bachmann BO, et al. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2012 Jun;153:1082-90.e2. Epub 2012 Mar 6.
- Kinoshita S, Koizumi N, Ueno M, et al. Injection of cultured cells with a ROCK Inhibitor for bullous keratopathy. N Engel J Med. 2018 Mar 15;378:995-1003.
- Rafat, M, Jabbarvand, M, Sharma, N. et al. Bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts. Nat Biotechnol. 2023;41:70- 81.
- Gain P, Jullienne R, He Z, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167-173.










