The eye is the window to the soul, and capturing the intricacies of the retina through advances in imaging technology allows improved identification and treatment of various pathologies. As research develops new treatments for diseases such as for geographic atrophy (GA), imaging modalities allow us to make the correct diagnosis and monitor response to treatment. New technologies, such as artificial intelligence (AI), also have the potential to change how we practice day-to-day.
In this article, I’ll discuss several imaging modalities used for AMD, GA and diabetic retinopathy (DR).
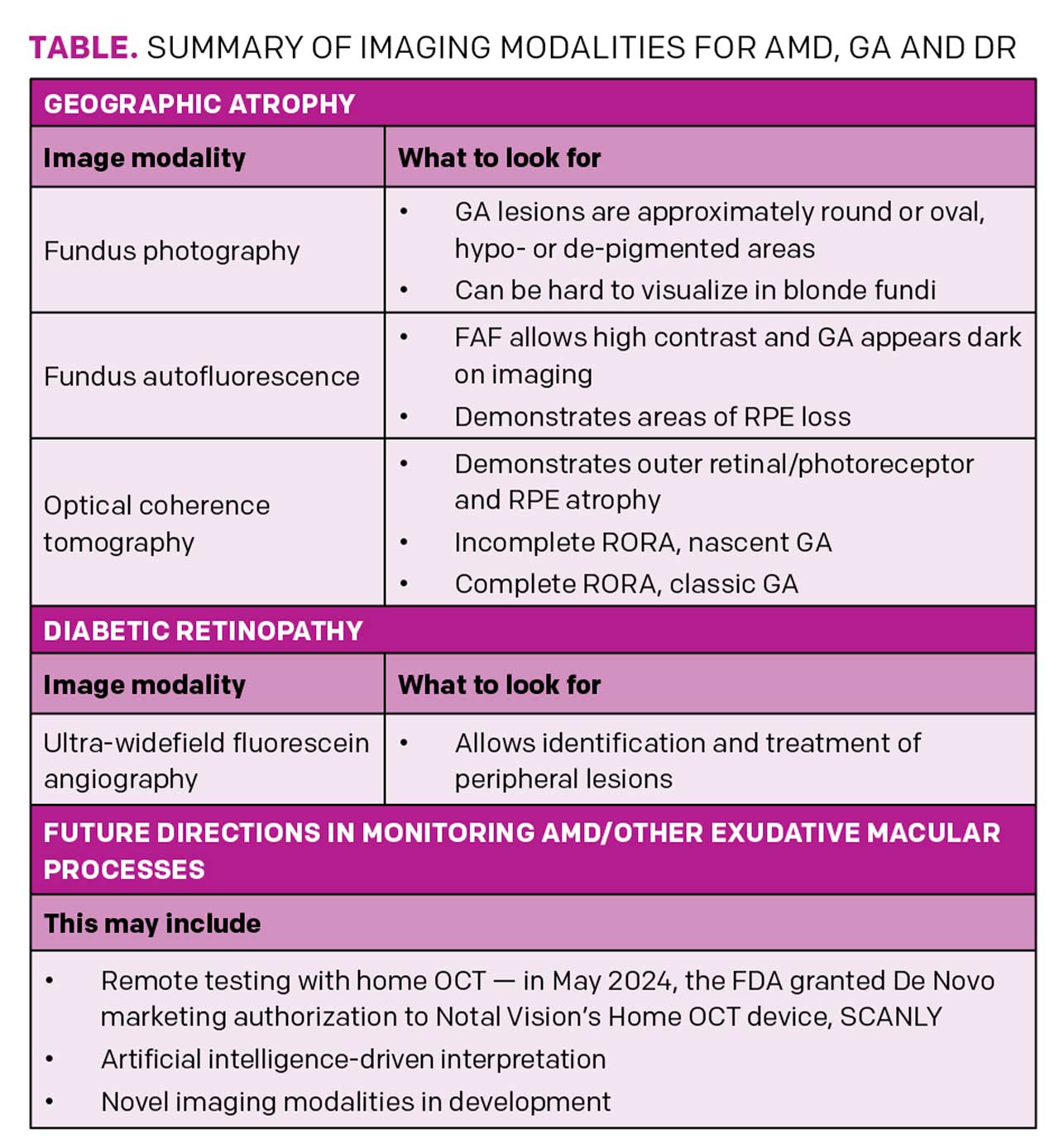
NONEXUDATIVE AMD/GEOGRAPHIC ATROPHY
GA is an advanced form of AMD with no treatment to reverse vision loss. It is the leading cause of permanent blindness in older adults in the western hemisphere.1 In early stages, the fovea is not involved. Symptoms may be absent but can include scotoma and metamorphopsia. When GA progresses to involve the fovea, profound vision loss may occur. Imaging modalities may aid in diagnosis but also in progressive changes with time, allowing for monitoring of response to therapy and improved discussion of prognosis.
Fundus photography
In color photography, GA lesions are approximately round or oval, hypo- or de-pigmented areas that allow increased visualization of the underlying choroidal vasculature. However, fundus photography may not delineate GA borders well due to poor contrast (Figure 1A).2
Fundus autofluorescence (FAF)
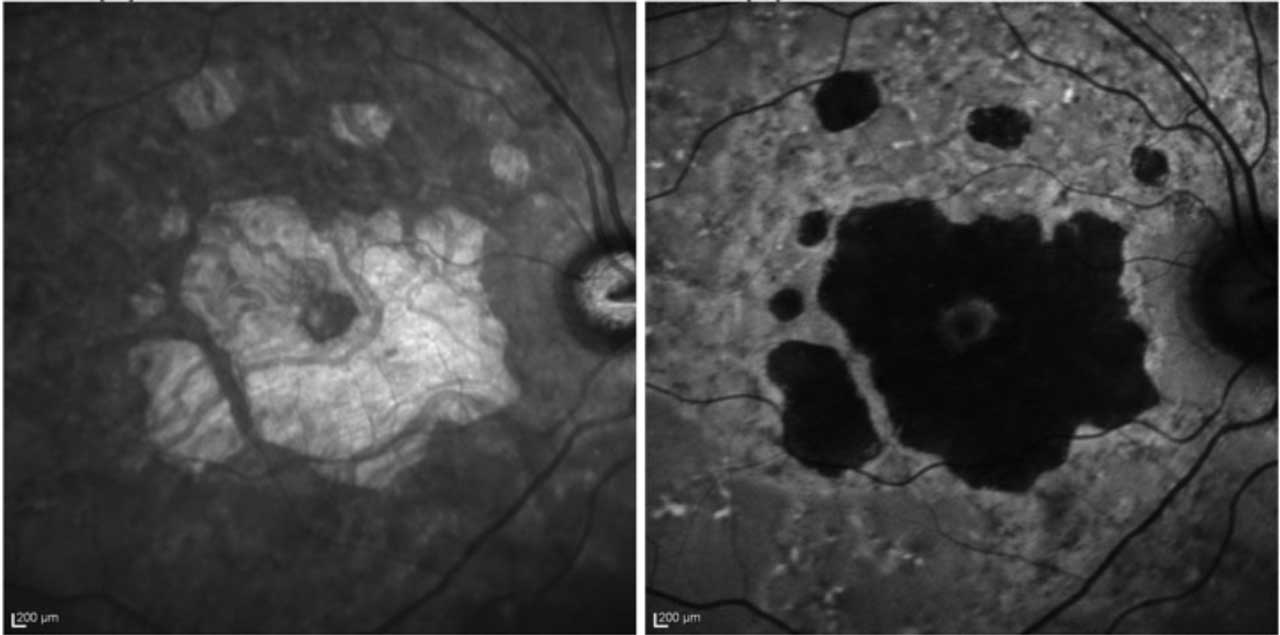
FAF allows high contrast of the GA border against the unaffected retina (Figure 1B). FAF captures the natural fluorescence of the lipofuscin in the retinal pigment epithelium (RPE). As this tissue atrophies, the signal attenuates and the GA appears dark on imaging.3 A reduction in the growth rate of GA area, as measured by FAF, was the primary endpoint for clinical trials of both currently available GA therapies.
Disadvantages include the lack of availability in some community centers. The light exposure of FAF is also more intense, with discomfort in some patients, and it temporarily bleaches the RPE, requiring a waiting period for repeat testing. Another common disadvantage is the blocked FAF at the fovea that can confound interpretation, especially in perifoveal lesions, as the macular luteal pigment also absorbs the excitation light of blue FAF.2 Green FAF or infrared testing can bypass this challenge.
Optical coherence tomography (OCT)
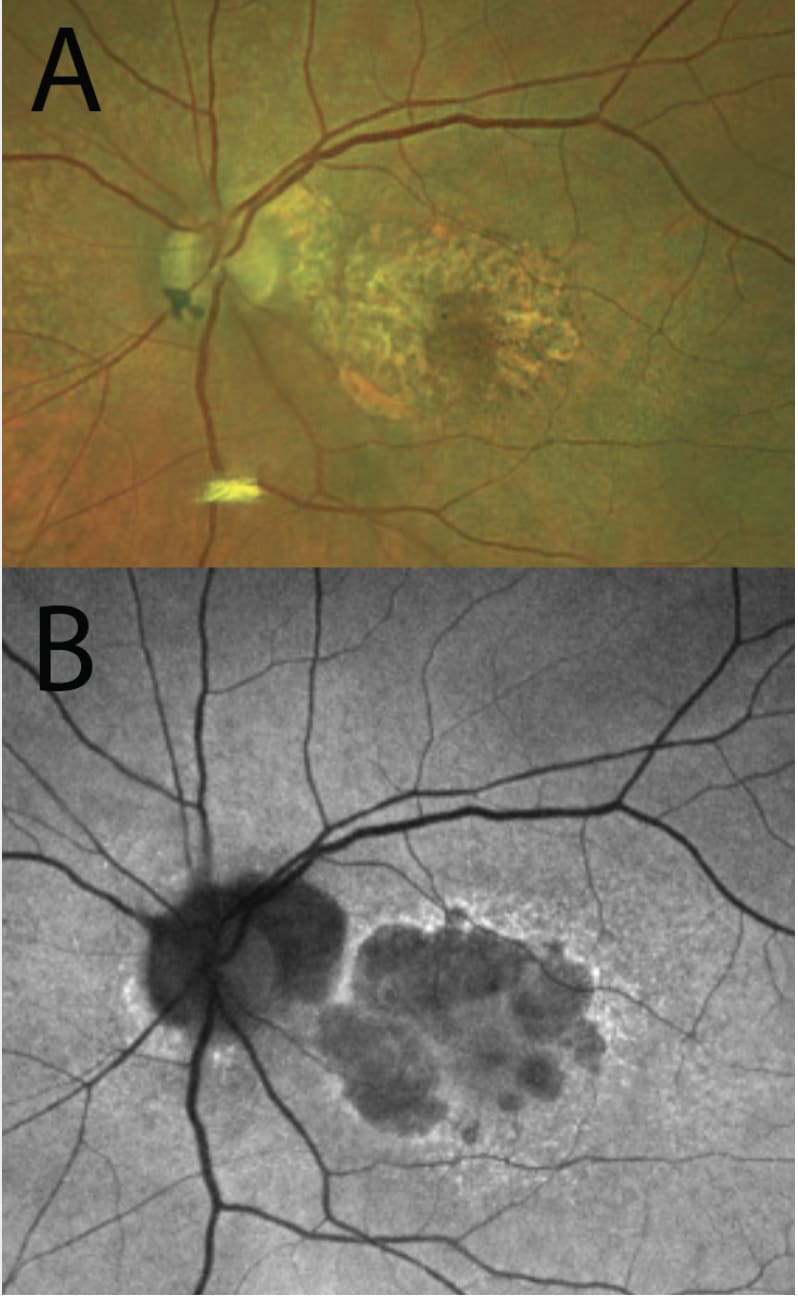
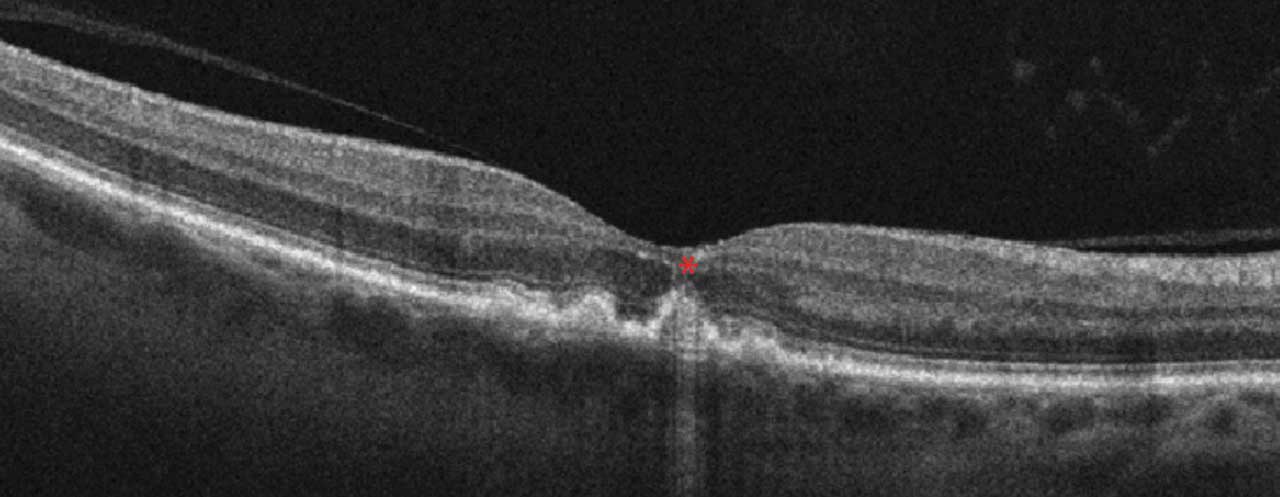
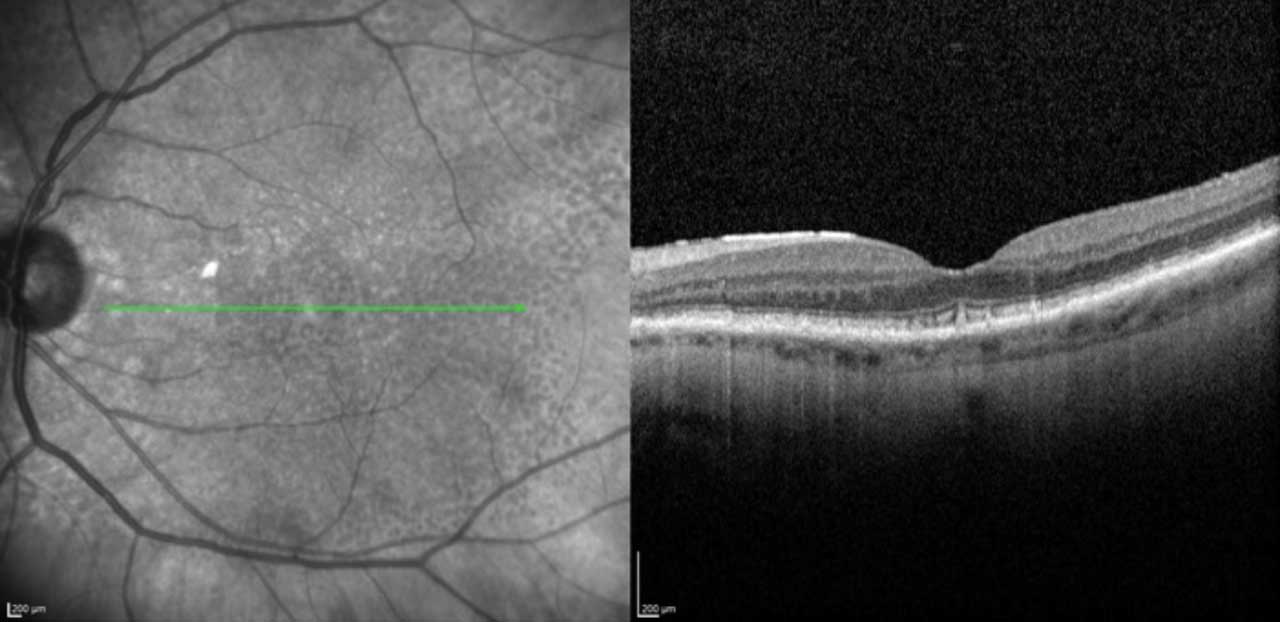
OCT is widely available and in common use by many ophthalmologists. Scans are fast and well-tolerated.2 In GA, increased signal continues below the retina and RPE into the choroid, termed hypertransmission.2
GA is described as RPE and outer retinal atrophy (RORA). These changes may be partial thickness and small, less then 250 µm, and called incomplete, or iRORA (Figure 2). Conversely, these changes may be larger than 250 µm and termed complete, or cRORA (Figure 3). When observed in the absence of a choroidal neovascular membrane (CNVM), iRORA is also called nascent GA, while cRORA is consistent with typical GA.2 iRORA is a high-risk feature of progression to cRORA.3 Measurement of photoreceptor loss is a primary outcome measure in ongoing clinical trials for GA therapeutics.
Infrared imaging
Infrared imaging is especially useful for the identification of reticular pseudodrusen, a risk factor for progression to late AMD (Figure 4). GA appears lighter than surrounding tissue (Figure 5), and imaging is more comfortable compared with FAF. However, this imaging modality is less established than others and requires further testing in large studies.1
EXUDATIVE AMD AND OCT
While fluorescein angiography (FA) and OCT angiography (OCTA) may confirm the presence and location of the CNVM, OCT remains the most common imaging modality in monitoring response to treatment in exudative disease. In wet AMD, fluid may be distributed through different retinal layers. Intraretinal fluid causes the most significant vision loss, as it represents disruption of the neurosensory retinal tissues. Subretinal fluid, however, may be better tolerated in some cases, such as in persistence of fluid in eyes undergoing anti-neovascular treatment. This finding may represent slowed drainage via the RPE, rather than true active exudation, and correlates with better vision in some studies.4
Future directions
Future directions in monitoring AMD and other exudative macular processes may include remote testing with home OCT and AI-driven interpretation. For example, Notal Vision’s Home OCT device, SCANLY, was recently granted De Novo marketing authorization by the FDA, and ongoing research has shown feasibility.5 As anti-neovascular agents with an extended duration of action become available, the ability to monitor patients at home may decrease overall clinic visits; further study is underway.
DIABETIC RETINOPATHY
Fluorescein angiography
While FA has been long established as a helpful evaluation tool for the diagnosis of DR, historical technology was limited to visualization over the posterior pole. Ultra-widefield (UWF) imaging allows identification of peripheral lesions. A recent DRCR study (Protocol AA) compared standard ETDRS FA with Optos 200Tx imaging. UWF FA identified more diabetic lesions than color photography,6 and visualization of peripheral lesions increased the DR severity score by 2 or more steps in more than 10% of studied eyes.7 Eyes with predominately peripheral lesions, as identified on UWF FA, were more likely to progress or require treatment. Thus, UWF FA may be a helpful modality at detecting clinically important DR lesions and prompting appropriate intervention.8
OCT angiography
OCTA may be most helpful in assessing macular ischemia in eyes of diabetic patients. OCTA parameters correlate well to stages of DR. Indeed, even patients with pre-diabetes have changes to the capillary vessel density before visible retinal vasculature changes occur. Treatment with anti-neovascular agents has generally been shown to have no effect on the foveal avascular zone area or macular vessel density.9
Compared with FA, OCTA has the benefit of being noninvasive. However, historically, imaging has been limited to macular assessment. UWF OCTA technology has been improving, though, allowing noninvasive detection of neovascularization with good correlation to FA results.9
Future directions
Fluorescence lifetime imaging ophthalmoscopy (FLIO) is a novel imaging technology in research that detects the amount of time a fluorophore remains in an excited state. Distinct patterns exist for different disease states, and imaging may provide an opportunity for earlier detection than current technology allows.10
Retinal oximetry is a novel, non-invasive assessment of retinal oxygenation by detecting the difference in optical properties between oxygenated and deoxygenated hemoglobin. Using this testing, retinal oxygenation has been shown to be greater in diabetics than non-diabetic controls, possibly related to arteriovenous shunting to bypass ischemic peripheral vasculature.10
Adaptive optics (AO) is an emerging imaging modality that allows high resolution scans, with visualization to the level of individual photoreceptor cells. This imaging technique works by reducing ocular aberrations and wavefront distortions, similar to telescopic visualization of distant stars through our atmosphere.
AI technology is already FDA approved for DR screening and telemedicine, with improvement in patient adherence while reducing unnecessary referrals. Future AI directions may include improved OCTA testing and interpretation.10
CONCLUSION
In the diagnosis and monitoring of GA, FAF allows a high contrast means to visualize RPE loss, while OCT may allow detection and monitoring of photoreceptor loss. New developments in AI may allow improved screening for DR and monitor for response to anti-neovascular treatments from a patient’s home.
Up-and-coming technologies, like FLIO and AO, may give us new understanding of retinal disease. OM
REFERENCES
1. Garrity ST, Sarraf D, Freund KB, Sadda SR. Multimodal Imaging of Nonneovascular Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2018;59:AMD48-AMD64.
2. Sadda SR, Guymer R, Holz FG, et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3 [published correction appears in Ophthalmology. 2019 Jan;126(1):177]. Ophthalmology. 2018;125:537-548.
3. Vujosevic S, Loewenstein A, O’Toole L, et al. Imaging geographic atrophy: integrating structure and function to better understand the effects of new treatments. Br J Ophthalmol. Published online January 30, 2024.
4. Rispoli M, Cennamo G, Antonio LD, et al. Practical guidance for imaging biomarkers in exudative age-related macular degeneration. Surv Ophthalmol. 2023;68:615-627.
5. Blinder KJ, Calhoun C, Maguire MG, et al. Home OCT Imaging for Newly Diagnosed Neovascular Age-Related Macular Degeneration: A Feasibility Study. Ophthalmol Retina. 2024;8:376-387.
6. Silva PS, Liu D, Glassman AR, et al. Assessment of Fluorescein Angiography Nonperfusion in Eyes With Diabetic Retinopathy Using Ultrawide Field Retinal Imaging. Retina. 2022;42:1302-1310.
7. Aiello LP, Odia I, Glassman AR, et al. Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-Field Imaging With Ultrawide-Field Imaging for Determining Severity of Diabetic Retinopathy. JAMA Ophthalmol. 2019;137:65-73.
8. Marcus DM, Silva PS, Liu D, et al. Association of Predominantly Peripheral Lesions on Ultra-Widefield Imaging and the Risk of Diabetic Retinopathy Worsening Over Time [published correction appears in JAMA Ophthalmol. 2023 Jan 1;141(1):104]. JAMA Ophthalmol. 2022;140:946-954.
9. Crincoli E, Sacconi R, Querques L, Querques G. OCT angiography 2023 update: focus on diabetic retinopathy. Acta Diabetol. 2024;61:533-541.
10. Nanegrungsunk O, Patikulsila D, Sadda SR. Ophthalmic imaging in diabetic retinopathy: A review. Clin Exp Ophthalmol. 2022;50:1082-1096.









