Since the first multifocal IOLs became available, refractive cataract surgeons have identified these lenses as excellent choices for the treatment of presbyopia in patients undergoing lens-based surgery. However, a significant problem with light-splitting presbyopia-correcting IOLs is their propensity to induce dysphotopsias. Although dysphotopsias have lessened with better technology, they remain a problem for many patients and are a rate-limiting barrier towards wider adoption. True accommodating lenses, alternatively, would be the future in the management of presbyopia.
One of the great disappointments in ophthalmology is that only one accommodating lens has received FDA approval in the 25 years since the first presbyopic IOL — and that lens is relatively ineffective and would never be approved today. Several other accommodating lenses were developed but all failed. The problem with the previous generations of accommodating lenses is their reliance upon vertex movement within the eye.
The good news today is that several accommodating IOLs in development mimic the natural accommodative process, a change in shape rather than a change in vertex position, and provide vision at distance and near without splitting light or inducing dysphotopsias. And most of these lenses may be available in the next few years.
The future of presbyopic cataract surgery will undoubtedly be accommodating IOLs. The following are just a few of the many accommodating IOLs in clinical development.
JUVENE
A modular, curvature-changing IOL, Juvene (LensGen, Inc.) improves vision at all distances, and outcomes remain stable 1 year after implantation. This novel IOL is comprised of a base lens that is supported by circumferential silicone haptics that fill the capsule, similar to the natural crystalline lens, and a fluid lens that fits into the base lens and facilitates a continuous range of focus from distance to near. The two lenses are modular and can be implanted through a relatively small incision. The silicone base lens goes into the capsular bag, and the refractive lens is placed on top of, and snapped into, the base lens in a two-part procedure. As ciliary muscle contraction occurs, fluid (silicone) fills the refractive component and causes a bulging of the lens and, thus, accommodation.
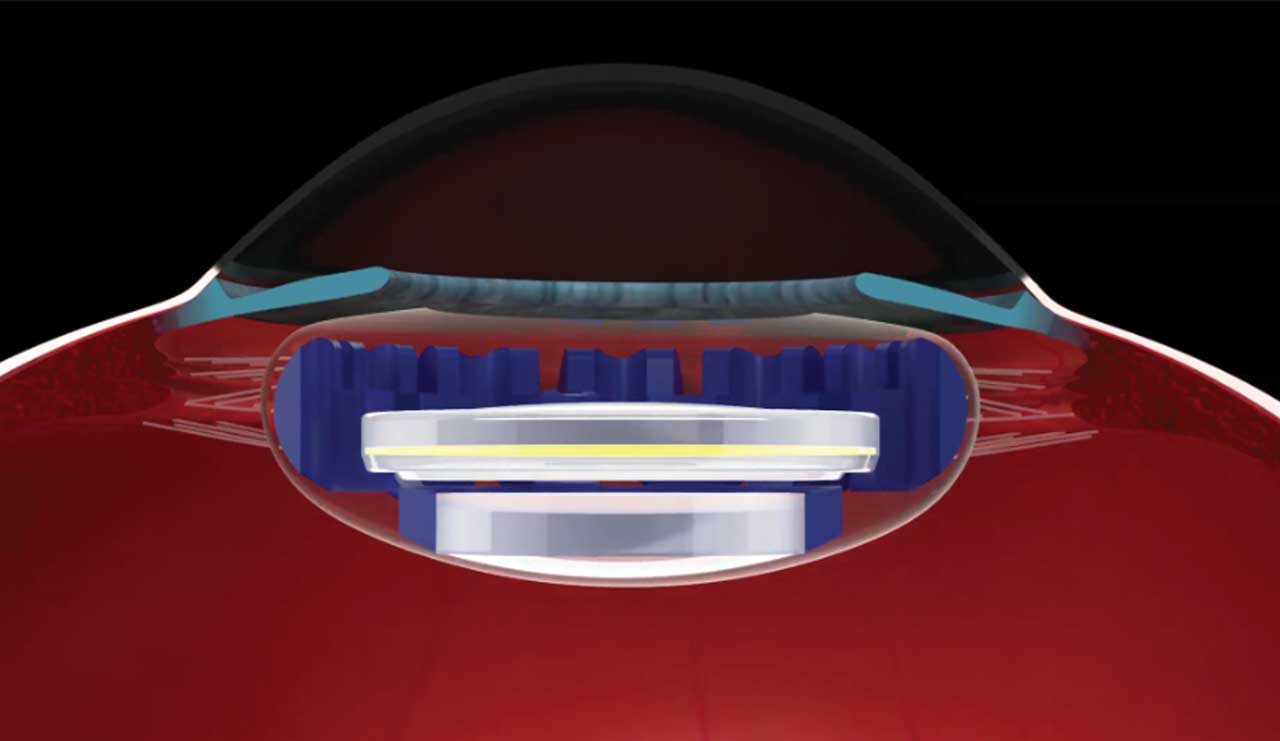
This mechanism offers several advantages — we can create a stable effective lens position and the capsular bag does not contract, making the refraction stable as well as reducing the vitreoretinal traction and lowering the risk of floaters postoperatively. Rotation of the IOL is minimal, and posterior capsular opacification is minimal to absent after 5 years.

The lens requires a standard phacoemulsification procedure that can be done manually or with a femtosecond laser. The phacoemulsification can be performed through a 2.4-mm incision followed by wound enlargement to 2.9 mm to 3.0 mm.
Following early prototypes, a final lens design was implanted into 54 eyes at two sites in Mexico in the Grail Study as an Investigational Device Exemption device. Eight surgeons performed the procedures. Data were also available from 15 cases of bilateral implantation. Thirty-six-month efficacy and safety data were presented at the 2023 ASCRS annual meeting.1 Monocularly, mean corrected distance visual acuity (CDVA) was 20/18, distance-corrected intermediate VA was 20/26 and distance-corrected near VA was 20/35, further improving with binocular summation.
The monocular defocus curve at the 6-month timepoint in the Grail Study showed that the IOL provided about 2.5 D of accommodation. When the Juvene IOL was implanted binocularly, about 3 D of accommodative power were achieved and confirmed by studies of reading and reading speed.
Contrast sensitivity with the Juvene IOL is identical to a normal phakic lens with minimal glare and halo similar to a monofocal IOL and significantly better than a multifocal IOL. A patient satisfaction survey completed by 15 patients reflected the results, with no visual disturbances, focusing difficulties or depth perception issues reported by any of the patients. The effective lens position and rotational orientation were stable at 36 months. In addition, there was no significant loss of corneal endothelium. Finally, after more than 4 years of follow-up, there was no posterior capsular opacification.
The FDA trial of the Juvene lens is expected to begin in 2024.
FLUIDVISION
The FluidVision lens (Alcon, formerly PowerVision) has an acrylic shell that is filled with silicone oil, allowing it to change shape with an accommodative amplitude of about 2.0 D. As the ciliary muscle contracts for near focus, fluid inside the haptics is pushed into the optic portion of the IOL, changing the curvature of the lens. The ciliary muscles relax to focus at distance, causing fluid to move back into the haptics, thus mimicking a natural lens with accommodation relaxed.2 The FluidVision lens has a one-piece design and functions much like the Juvene IOL. The lens in its current form requires a 3.2-mm incision.
The ORION study is a prospective, multicenter trial comparing the FluidVision IOL with the AcrySof IQ IOL (Alcon). The study has shown excellent distance-corrected intermediate and near visual acuity, contrast sensitivity similar to the monofocal lens, and an objective amplitude of accommodation of up to 2.20 D.
OMNIVU
The OmniVu (Atia Vision) accommodating lens is a dual-optic modular IOL. It features a shape-changing, fluid-filled base and a fixed-power front optic. The front optic is made from a hydrophobic acrylic material, with three docking tabs around the 5.6-mm optic. The shape-changing fluid-filled base shell changes power with ciliary muscle contraction and has an inner channel, which allows for the docking of the fixed power front optic. With attempted accommodation, fluid moves from the periphery of the base toward its center, changing the shape and increasing the power of the optic.
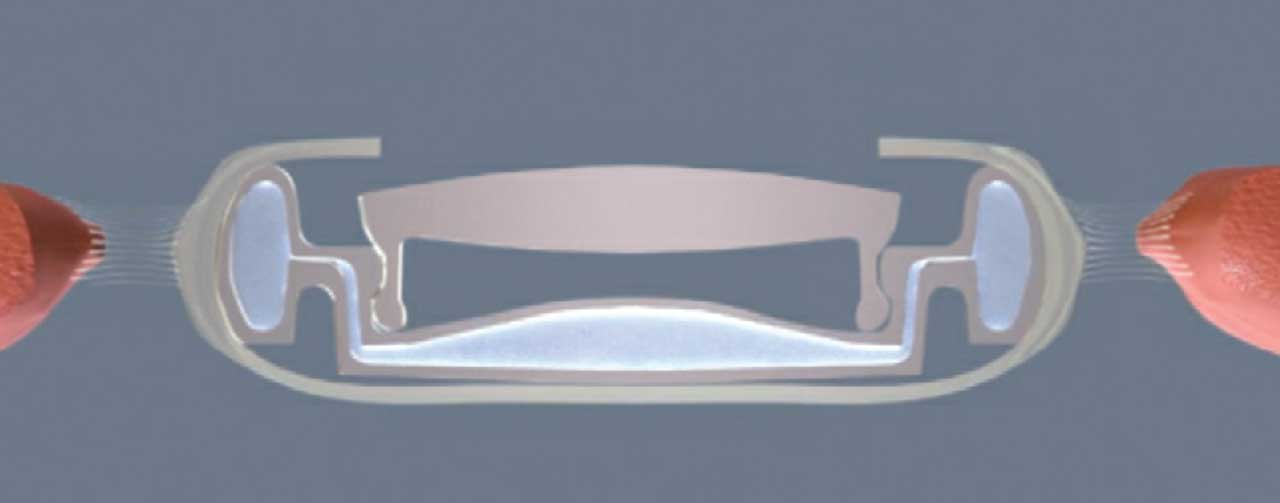
Early results showed that 95% of eyes were within 0.5 D of emmetropia 6 months postoperatively.3 All patients achieved binocular CDVA of 20/20 for distance and intermediate and 20/32 for distance-corrected near. The patients maintained greater than 20/32 across 4 D of defocus binocularly, and refractive predictability and stability were stable.
The base lens is inserted through a 3.5-mm incision and into the capsular bag after a normal phacoemulsification procedure with an intended 5.5-mm anterior capsulotomy. Then, the fixed power front optic lens is injected into the capsular bag and docked into the base.
LUMINA
The Lumina (AkkoLens) is a hydrophilic acrylic lens designed for positioning at the sulcus plane.4 The lens consists of two optical elements that each have an elastic omega-shaped loop with a spring function and non-elastic connections to the main body of the lens. When the lens is positioned in the sulcus plane, the ciliary muscle contacts the body of the lens and drives accommodation directly. The two optical elements slide in a plane perpendicular to the optical axis and produce a continuous variable focus.
In the unaccommodated state, the omega-shaped springs of the Lumina relax, the optical elements overlap, and the lens displays its lowest optical power. When the eye attempts accommodation, the ciliary muscle contracts and compresses the Lumina, resulting in a mutual shift of the optical elements. Optical power increases linearly with the shift, and the eye focuses at closer distances. The size of the Lumina is dependent on the size of the eye and must be customized to the diameter of the ciliary body.

The Lumina received the CE Mark and is available in Europe. Two multicenter studies of the lens are currently underway.
Eighty-six eyes were enrolled in a randomized clinical trial and observed for 1 year.4 The study group included 61 eyes that received a Lumina lens, and the control group included 25 eyes that received an AcrySof SA60AT monofocal IOL (Alcon). Uncorrected distance visual acuity (UDVA), CDVA and contrast sensitivity did not differ significantly between the groups during the study period. Uncorrected near visual acuity (UNVA) and distance-corrected near visual acuity, however, were significantly better in the Lumina group. Defocus curves showed a statistically significant difference between groups for defocus ranging from -4.50 to -0.50 D, with significantly better visual acuities for the Lumina group.
Subjective accommodation, as determined from defocus curves, was 3.05 ±1.06, 3.87 ±1.27, and 5.59 ±1.02 D for the Lumina group and 1.46 ±0.54, 2.00 ±0.52, and 3.67 ±0.75 D for the control.
OPIRA
The Opira accommodating IOL (ForSight Vision6) consists of two elements that are implanted separately: a static posterior lens and a dynamic anterior surface. The IOL’s haptics are fixated to the capsulorrhexis, so capsulorrhexis size needs to be precise. For this reason, this IOL will likely require the precision of a femtosecond laser or precision pulse capsulotomy (Zepto, Centricity Vision) to accurately support the implant. This system allows for direct interaction between the ciliary body and the lens by not having the IOL positioned within the capsular bag. An early clinical trial of 16 patients found the lens achieved an UNVA equivalent of 20/25.

In in-vitro optical tests, the Opira lens sustained a monofocal quality of vision across all ranges of defocus. Two years of clinical follow-up data are available for a cohort of 29 patients who received a monofocal IOL in one eye and the Opira in the contralateral eye. The eyes that received the Opira had better distance-corrected intermediate and near visual acuity than the eyes that received the monofocal lens. Posterior capsule opacification, which occurred in many eyes, was treated by Nd:YAG laser posterior capsulotomy without negative effects on accommodative function. Uveitis-glaucoma-hyphema syndrome has not been observed with the lens, because it remains stable in the eye and the lens’ surfaces are smooth (data on file with ForSight Vision6).
The Opira can be inserted through a 3.9-mm clear corneal incision and, with planned design changes, there is hope it can be implanted through a smaller incision.
AUTOFOCAL SAPPHIRE
An IOL in previous development, which is not currently active but remains exciting, is the electroactive AutoFocal Sapphire IOL (Elenza). It is the first lens that allows for visual accommodation without movement of the lens. This lens creates accommodation through an electro-active liquid-crystal optic that is encapsulated within a high-quality aspheric monofocal IOL. The lens contains a photovoltaic cell with photo sensors that monitor the patient’s pupil dynamics associated with accommodation and a solid-state rechargeable power cell.
PREPARE YOURSELF
Many of the accommodating IOLs in development rely on a posterior capsule that is well centered and associated with strong zonules. Femtosecond lasers and precision pulse capsulotomy devices certainly help with this process and may be mandatory for certain lens designs.
In addition, for many lenses to work in the long term, the capsule must remain clear and flexible without fibrosis or contraction. One of the valuable aspects of these accommodating IOLs is that by maximally filling the capsular bag and separating the anterior and posterior aspects of the capsule, the posterior capsule remains clear. In addition, there is less risk of forward vitreous expansion creating vitreous floaters.
Finally, these lenses may have one- or two-piece technologies. Regardless, they are larger, more flexible and require new injectors and surgical skills that, while not daunting, will be different than those required for the conventional IOLs we utilize today.
These lenses are essentially monofocal lenses and offer the possibility of clear vision at near and far without dysphotopsias. For this reason, accommodating IOLs remain the holy grail of presbyopia correction.
Ophthalmologists interested in offering the best refractive options to their patients, providing services and technologies that will not likely be covered by traditional governmental and private health insurance companies will want to stay abreast of developments in the accommodating lens arena, possibly even becoming involved in clinical trials of these devices. They may also want to become comfortable using femtosecond lasers if they are not already. OM
References
1. Garg S. Thirty-six-month visual outcomes after implantation of a new modular, shape-changing, fluid-optic intraocular lens. Paper presented at: Annual meeting of the American Society of Cataract and Refractive Surgery; May 5-8, 2023; San Diego, CA.
2. Kent C. Accommodating IOLs: two more possibilities. Review of Ophthalmology. Published December 11, 2019.
3. Waring GW. Prospective, open-label, non-randomized trial of a presbyopia-correcting, modular intraocular lens system for treatment of cataract. Paper presented at: Annual meeting of the American Society of Cataract and Refractive Surgery; May 5-8, 2023; San Diego, CA.
4. Alio JL, Simonov A, Plaza-Puche AB, et al. Visual outcomes and accommodative response of the Lumina accommodative intraocular lens. Am J Ophthalmol. 2016;164:37-48.










