Glaucoma is a chronic and slowly progressive disease; most patients suffering from this condition will require a variety of topical, laser and interventional approaches to successfully manage the disease throughout their lifespan.
While glaucoma has traditionally been a disease where topical pharmacological therapy was the primary and secondary modes of treatment, the paradigm is shifting as a result of recent data.1 The LiGHT study was a prospective randomized trial in which patients who underwent a laser trabeculoplasty had less visual field progression and required fewer surgical interventions than those taking topical therapy (usually latanoprost). Additionally, two major approaches have evolved over the past decade: microinvasive glaucoma surgery as well as the first extended-delivery glaucoma therapy, sustained-release bimatoprost (Durysta, Allergan).2
Fortunately, we now have categories of glaucoma therapy that can be offered by the physician in the office or minor procedure room environment. In this article, we discuss some common in-office glaucoma procedures that we perform on a regular basis, along with a few thoughts or tips on how to optimize and efficiently provide these procedures for our patients.
PROCEDURES
Selective laser trabeculoplasty
While most physicians perform selective laser trabeculoplasty (SLT) as part of residency training, we thought we could add a few helpful pointers. We typically treat for 360°, 100 pulses at 0.7 or 0.8 mJ. The COAST trial (www.coasttrial.org/goals/ ) is investigating low dose (0.4 mJ), annual laser, but until that study is finalized, a traditional SLT dosage seems reasonable. The dose-response curve for SLT is currently not well characterized.3
The LiGHT trial gave us the confidence to perform this procedure as the first treatment for primary open-angle glaucoma patients. In cases where gonioscopic view is suboptimal (corneal scarring, peripheral anterior synechia), we avoid performing traditional SLT. Alternatively, these patients may be candidates for transscleral SLT, which is 1.4 mJ delivered directly to the trabecular meshwork through the limbus (no lens needed).4 BELKIN Vision’s Eagle laser currently delivers trans-scleral or direct SLT and may soon be available in the United States, as it is currently available in Europe.
Durysta
We perform this procedure at the slit lamp or laser with or without an island speculum (Figure), depending on whether the patient is likely to squeeze. Topical tetracaine and betadine are given prior to placement. Durysta is then placed into the anterior chamber and inserted. In pseudophakic patients, it is reasonable to administer Durysta into the iridociliary sulcus through the pupil.
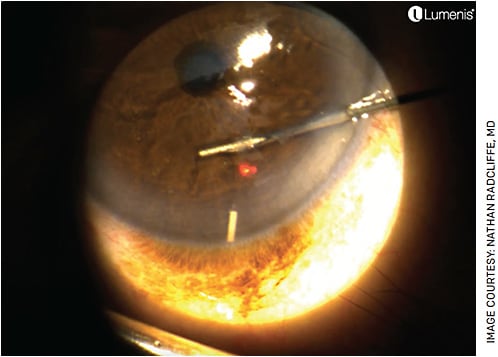
We frequently perform bilateral same-day injections, as we find most patients see normally after the procedure and prefer the efficiency of having both eyes treated on the same day. Allergan offers Allergan EyeCue, which is a comprehensive resource that assists with patient management and reimbursement support for physicians and their patients. One can request benefit verifications and gain access to savings programs.
Paracentesis
In some situations, for patients with an acutely elevated IOP, it makes sense to withdraw fluid from the anterior chamber to temporarily reduce the pressure. The following is a tip from Paul Palmberg, MD, PhD, of Bascom Palmer: After tetracaine and betadine, when a 30-g needle is placed on a 1-cc syringe (with the plunger removed) and is placed through the peripheral cornea, the IOP will typically go down to 6 mm Hg but not lower due to the internal resistance to outflow from the 30-g needle.
We usually deliver additional topical therapy to the eye following the paracentesis, as the starling forces associated with the lower IOP are more accommodating to the IOP-lowering drops.
Bleb needling
While physicians that perform bleb needlings are likely to already have a preferred technique, here are a few suggestions for needling tube shunt blebs. Encapsulation of the tube shunt bleb is common, particularly following a valved tube shunt, and serial paracentesis of the tube capsule as described above can also be helpful. Jeffrey Freedman, MD, of SUNY Downstate is credited with the recognition that the aqueous humor cytokines in the hypertensive bleb are in some manner contributing to the encapsulation.5 We will commit to this treatment when we see an elevated bleb over an Ahmed valve plate in the first few months postoperatively.
The first time the procedure is performed, following tetracaine and betadine, a 25-g needle on a syringe hub with no plunger is placed into the bleb, and one will likely see 0.2 or 0.3 cc of aqueous enter the syringe as the bleb size is reduced. The IOP will be lower following this procedure. This confirms that the tube is working anatomically and that encapsulation is the problem. This procedure can be repeated weekly until the IOP normalizes. The approach can be dramatically effective.
Later on, perhaps more than 6 months after the valve is placed, some of our colleagues will needle the capsule surrounding the tube, as one would a trabeculectomy bleb, often injecting and antimetabolite at the end of the procedure. While this is likely to be successful less than half of the time, it is a reasonable procedure to offer prior to performing a diode laser or returning to the operating room for an additional shunt.
Anterior chamber reformation
Surgeons who perform enough tube shunt surgeries will eventually need to reform a shallow or flat anterior chamber due to hypotony within the first few postoperative weeks (prior to tube plate encapsulation). The good news is that anterior chamber reformation with viscoelastic can fight off choroidal effusions dramatically, reducing the need to have choroidal effusions drained surgically.
After the tetracaine is instilled and betadine is applied, we place a 30-g needle on a syringe and inject a copious amount of a cohesive viscoelastic into the anterior chamber (basically as much as we can manage), especially if choroidal effusions are present. The anterior chamber should be hyper deep after the injection. Remember that as the IOP comes up and the effusions shrink, the volume of the eye will increase, making more room for the viscoelastic and dropping the IOP again. The goal is to leave these eyes with a pressure of 25 mm Hg or so.
Tube revision/repositioning
Tubes can be modestly revised at the slit lamp or in the office setting in a few select situations. When iris is clogging a tube, a needle with Provisc (Alcon) in the syringe can be used to sweep iris out of the tube tip (warning, the patient may feel some discomfort). When a sulcus tube has iris incarceration, I will see an elevated IOP and an iris “dimple” as the presenting sign, and a sweep behind the iris with a 30-g needle may alleviate the problem. Following this intervention, surveillance for recurrence must be employed, and in some cases, an iridotomy in the location of prior incarceration can be helpful.
TIPS FOR STARTERS OF IN-OFFICE PROCEDURES
Environment
Glaucoma physicians should have various policies and guidelines (with respect to set-up, sterility and minor OR environment) established in terms of set-up when performing office procedures; however, a minor procedure room may be preferred depending on the nature of the procedure.
When performing procedures in office, most can be done at the slit lamp with some experience. The slit lamp should be stable, functional and ergonomically adequate for both the physician and patient. Unstable slip lamps that either move or cannot be locked into place are less preferable. Performing procedures at a laser slit lamp has its advantages, as lasers can handle a heavy load and have wheels that can be locked completely.
Once an adequate environment for the procedure is established, a few universally helpful tools can assist.
About cost and reimbursement
In these challenging times in healthcare, cost and reimbursement are always important factors to consider. In terms of medical supply expenses in the office for the above-mentioned procedures, the supplies needed are fairly simple and inexpensive. General surgical equipment and ophthalmology-specific suppliers will most likely have everything one needs.
Concerning anesthetics, we prefer tetracaine due to its efficacy, and some form of lidocaine gel is usually helpful as well. Topical betadine (5%) should be applied to any eye undergoing a penetrating procedure, whether it be a viscoelastic or medication injection or bleb needling.
While all of the above procedures are performed in the office by the surgeon, some are stand-alone procedures, like the SLT (CPT code 65855) and Durysta implants (CPT code 66030 and HCPCS code J7351), and others are a result of surgical procedure or, in some cases, complication. Some procedures that may occur within the postoperative period are not included in the global surgical period and can be separately billed.
Modifiers can be used on claims for reimbursement for procedures that occur during the global surgery period (eg, paracentesis). For example, modifier 58 is described as a staged or related procedure or service by the same physician or other qualified health-care professional during the postoperative period. Diagnostic testing performed to confirm the expected results of the surgery are considered to be part of routine postoperative care and should not be billed separately, whereas tests to evaluate and plan the management of a complication can be separately billed.
Well-documented medical records are a key component when dealing with payors in the event of pre- and post-payment audits.
CONCLUSION
In 2023, we are fortunate to have so many options for assisting our patients in their lifelong journey with glaucoma. A variety of procedures can be performed in the office to assist glaucoma patients throughout the spectrum of disease. OM
REFERENCES
- Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505-1516.
- Medeiros FA, Walters TR, Kolko M, et al. Phase 3, Randomized, 20-Month Study of Bimatoprost Implant in Open-Angle Glaucoma and Ocular Hypertension (ARTEMIS 1). Ophthalmology. 2020;127:1627-1641.
- Radcliffe N, Gazzard G, Samuelson T, et al. Energy Dose-Response in Selective Laser Trabeculoplasty: A Review. J Glaucoma. 2022;31(8):e49-e68.
- Geffen N, Ofir S, Belkin A, et al. Transscleral Selective Laser Trabeculoplasty Without a Gonioscopy Lens. J Glaucoma. 2017;26:201-207.
- Freedman J. Tube-Shunt Bleb Pathophysiology, the Cytokine Story. J Glaucoma. 2021;30:109-113.










