OPHTHALMOLOGISTS FACE 8.5% CUT IN REIMBURSEMENT
CMS final rule for 2023 would reduce physician reimbursement, increase ASC and hospital fees.
BY JAMES D. GALLAGHER, CONTRIBUTING EDITOR
Ophthalmologists will see a reduction in Medicare reimbursement in 2023, while hospitals and ASCs will receive a slight increase in payment for surgical procedures, according to the Physician Fee Schedule (PFS) final rule issued Nov. 1, 2022, by the Centers for Medicare and Medicaid Services (CMS).
Under CMS’s final rule, the conversion factor (CF) used to determine physician reimbursement will drop by 4.47% in 2023, from $34.61 to $33.06. The CF, which federal law requires to be updated annually, is part of a complex formula used to determine how much Medicare will pay for medical procedures. This includes both facility fees and nonfacility costs that are reimbursible.
In a press release, CMS explained that the CF reduction is due to federal budget neutrality adjustments, as well as the expiration of a 3% supplemental increase to PFS payments for 2022 that was authorized by the Protecting Medicare and American Farmers from Sequester Cuts Act of 2021. When coupled with a 4% Medicare cut that is required under the 2010 Statutory Pay-As-You-Go (PAYGO) Act, physicians will face a nearly 8.5% Medicare cut beginning on Jan. 1, 2023.
ADVOCACY GROUPS OPPOSE MEDICARE REDUCTION
Physician advocacy groups have strenuously opposed the Medicare reduction, citing inflation of roughly 8% over the past 12 months and the effects of the “Great Resignation,” which has forced many medical practices to pay more to attract and retain quality staff.
“The final rule comes amid surging medical inflation and staff retention challenges practices are experiencing across the country,” said George Williams, senior secretary for advocacy for the AAO, in a statement. “As the value of Medicare physician payments continues to plummet on an inflation-adjusted basis, the cuts will further diminish the financial support which surgical practices around the country rely on at a time when they need it most.”
ADVOCACY GROUPS REQUEST TWO FIXES
Consequently, the AAO, American Medical Association, American College of Physicians and other advocacy groups have encouraged Congress to pass two pieces of legislation that would alleviate the cuts: H.R. 8800, the Supporting Medicare Providers Act of 2022, and H.R. 3173/S. 3018, the Improving Seniors’ Timely Access to Care Act. The PAYGO Act requires changes to be approved by Congress 15 days before the end of the legislative session to reverse the Medicare reimbursement cuts.
SOME POSITIVE NEWS FOR OPHTHALMIC SURGEONS
The news from CMS was not all bad for ophthalmic surgeons, as the PFS final rule for 2023 will increase Medicare payment rates for cataract, retina and glaucoma procedures performed in an ASC by 3.8% across the board. This increase is higher than the 2.7% CMS projected in its initial draft of the PFS rule, issued in July 2022. Medicare reimbursement for hospital procedures would also increase by 3.8% in 2023. These fee changes and other policies in the CMS final rule will affect approximately 3,500 hospitals and approximately 6,000 ASCs.1
CMS REJECTS IN-OFFICE PROPOSAL
In issuing its final rule for 2023, CMS rejected a proposal that would have established facility payments for cataract, retina and glaucoma surgeries performed in physician offices rather than in ASCs or hospitals. During a comment period prior to release of the final rule, ophthalmic advocacy groups, including the AAO, ASCRS, the American Society of Retina Surgeons, Ophthalmic Outpatient Surgery Society (OOSS) and Ambulatory Surgery Center Association, opposed the office-based surgery proposal.
“CMS’s ruling [on office-based surgery] is a victory for patients,” says Jeffrey Whitman, MD, the chairman of government affairs for OOSS. “It reflects the concerns of OOSS and all the major ophthalmology organizations that cataract, retina and glaucoma patients are vulnerable and should be treated in an appropriately regulated environment like the ASC or hospital outpatient department.
“Regulation of [in-office] surgical facilities at the state level is non-existent at worst and inadequate and inconsistent at best,” Dr. Whitman says. “CMS’s ruling reflects the view that, to ensure patient health and safety, any facility that is conducting sterile intraocular procedures should be required to meet the same standards as those required for Medicare certification.” OM
REFERENCE
- CY 2023 Medicare Hospital Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System Final Rule with Comment Period (CMS 1772-FC). bit.ly/3AUFRUn . Accessed November 29, 2022.
OPHTHALMOLOGY ENTERS THE METAVERSE
The Digital Ophthalmic Society utilizes this virtual platform to take education and collaboration to another level.
BY JULIE GREENBAUM, ASSOCIATE EDITOR
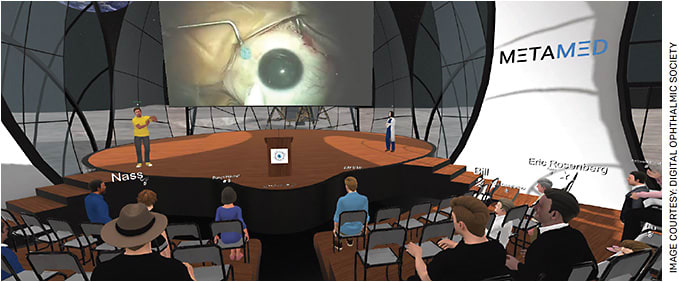
Imagine a future in which virtual conferences mirror the format of traditional conferences. Rather than walking around a convention center and sitting in a lecture hall, attendees use a virtual avatar and interact and learn from the best surgeons in the world virtually. They view surgical videos in stereoscopic 3D mode, hold grand rounds, have one-on-one private discussions using 3D spatial audio with the ability to play back the content in 3D, and move around by taking a virtual elevator or by transporting to a specific location.
In the Digital Ophthalmic Society (DOS), that future is now, and it’s taking place in the metaverse.
Eric Rosenberg, DO, MSE, SightMD, Plainview, N.Y., and co-founder of the DOS, Ranya Habash, MD, and Steven Houston, MD, FASRS, Florida Retina Institute, created MetaMed Media, a company that is built around the metaverse platform. The DOS and the rest of the health-care industry can use this platform to hold meetings in this immersive space.
“I was watching this technology evolve over the last several years and realized the tremendous benefit it could have for ophthalmologists. I contacted Steve and Ranya, established pioneers in this space, and we started working together to build this out,” says Dr. Rosenberg.
A PRACTICAL SOLUTION FOR THE BUSY OPHTHALMOLOGIST
The DOS offers three ways to attend a virtual meeting: with a virtual reality headset (Oculus, Pico Neo or VIVE) to experience the 3D video content and meet in a completely immersive environment; with a laptop or desktop computer for a 2D 4K HD experience; or with your iPhone/iPad.
Dr. Rosenberg says that the metaverse provides an alternative for MDs where they don’t have to choose whether to give up a clinic or day to travel to a meeting.
“Not everyone can go to meetings all of the time, so this gives ophthalmologists from around the world an alternative and the ability to attend a meeting without having to travel,” Dr. Rosenberg explains. “Being able to have a true virtual offering that replicates real life is going to be really valuable, especially when balancing the obligations of everyday life.”
While he doesn’t think that the metaverse is going to replace live meetings, Dr. Rosenberg says it will augment them and could even replace other virtual formats.
TYPES OF MEETINGS HELD IN THE METAVERSE
The DOS holds weekly RetinaVerse meetings that feature well-known retina surgeons from institutions such as Bascom Palmer Eye Institute, Duke University, Mass Eye and Ear and Stanford University, who have impromptu discussions about retina cases. The first meeting in 2022, for example, focused on doing straight up vitrectomies, and the second meeting focused on doing secondary IOLs.
Additionally, the DOS holds quarterly metaverse meetings — the first meeting in 2022 highlighted digital visualization, and the second meeting highlighted artificial intelligence (AI) and the integration in ophthalmology.
“Ophthalmology is a very three-dimensional field; the capability of being able to convey points and showcase novel techniques and use three dimensions in playback has been surreal,” Dr. Rosenberg notes.
PLANS FOR THE FUTURE
After initial positive reception, Dr. Rosenberg says that plans are currently in the works to also start a CorneaVerse meeting. Additionally, he and his team plan to expand the platform even further. “The platform is currently in talks with Microsoft and Meta and other technology-driven companies because the use case here is significant, especially for what we’re able to accomplish for our doctors,” he says.
Follow the DOS on LinkedIn, Facebook and Twitter and visit www. digitalophthalmicsociety.com to join the community. OM
IN THE NEWS:
IVERIC bio announced the FDA granted Breakthrough Therapy designation for avacincaptad pegol (ACP, also known as Zimura), a novel investigational complement C5 inhibitor for the treatment of geographic atrophy secondary to AMD. To date, ACP is the first investigational therapy to receive Breakthrough Therapy designation status for this indication.
AEYE Health received 510(k) clearance from the FDA to market its diagnostic screening system, the AEYE-DS, for diabetic retinopathy. The AEYE-DS is indicated for use with images obtained by the Topcon NW-400 desktop retinal camera.
Tarsus Pharmaceuticals’ new drug application (NDA) was accepted by the FDA for TP-03 (lotilaner ophthalmic solution, 0.25%) for the treatment of Demodex blepharitis, with a Prescription Drug User Fee Act target action date of Aug. 25, 2023.
Outlook Therapeutics announced the FDA accepted for filing a biologics license application for ONS-5010/LYTENAVA (bevacizumab-vikg), an investigational ophthalmic formulation of bevacizumab for the treatment of wet AMD. The FDA set a Prescription Drug User Fee Act goal date of Aug. 29, 2023.
Eyenovia announced that the FDA accepted for review its NDA for MydCombi ophthalmic spray. MydCombi is a drug-device combination product that comprises the company’s proprietary, first-in-class combination of tropicamide and phenylephrine for in-office pupil dilation (mydriasis), administered via the investigational Optejet drug delivery technology.
Oyster Point Pharma entered into a definitive agreement under which Viatris Inc. would acquire Oyster Point Pharma. Viatris says it intends to acquire Oyster Point Pharma as the foundation of its new ophthalmology franchise, recognizing its team, the strength of TYRVAYA (varenicline solution) Nasal Spray and the company’s pipeline.
Aldeyra Therapeutics announced the submission of a NDA to the FDA for topical ocular reproxalap, an investigational new drug (IND) candidate for the treatment of signs and symptoms of DED. In addition to DED, reproxalap is in late-stage development for allergic conjunctivitis, a condition that is commonly associated with DED.
Kala Pharmaceuticals announced the submission of an IND application to the FDA for KPI-012 for the treatment of Persistent Corneal Epithelial Defect (PCED). Subject to acceptance of the IND by the FDA, Kala remains on-track to initiate a Phase 2b clinical trial of KPI-012 for PCED in the fourth quarter of 2022.
Azura Ophthalmics Ltd. reported positive 3-month efficacy and safety results from its Phase 2b study of AZR-MD-001 0.5% in meibomian gland dysfunction. The trial met its co-primary endpoints of significant improvements in Meibomian Glands Yielding Liquid Secretion score, with patients experiencing an average increase of 1.8 more open glands secreting meibum from baseline (p = 0.0004).
OKYO Pharma filed an IND application with the FDA for the development of OK-101 to treat DED. Both nonclinical and clinical development plans on OK-101 were reviewed with the FDA in an earlier Pre-IND meeting with the FDA agreeing to a first-in-human Phase 2 trial in DED patients.
The FDA has accepted Apellis Pharmaceuticals’ unsolicited major amendment to the NDA for intravitreal pegcetacoplan for the treatment of GA secondary to AMD. The updated Prescription Drug User Fee Act goal date is Feb. 26, 2023.
Nicox SA announced that once daily dosing of NCX 470 0.1% met the primary objective of non-inferiority in lowering IOP in the 691-patient Mont Blanc Phase 3 clinical trial in patients with open-angle glaucoma or ocular hypertension. The IOP-lowering effect from baseline for NCX 470 was 8.0 mm Hg to 9.7 mm Hg.
Vyluma Inc. reported topline results from its Phase 3 CHAMP clinical study. Analysis of this study demonstrates strong safety and efficacy for NVK002, an investigational, low-dose, preservative-free atropine eyedrop, as a potential treatment for the progression of myopia in children. NVK002 at a dose of 0.01% atropine achieved significant and clinically meaningful differences from placebo in every key outcome measure.
Neurotech Pharmaceuticals shared positive results from two replicative Phase 3 trials with its cell therapy for the treatment of Macular telangiectasia type 2 (MacTel). The studies were designed to evaluate the safety/efficacy of the NT-501 implant. Results demonstrated change in the rate of progression of disease in patients in two Phase 3 trials: 56.4% rate of reduction in Protocol A and 29.2% rate of reduction in Protocol B.








