Separately reimbursed drugs need modifiers starting this year.
In calendar year (CY) 2023, the CMS is changing the way practices and facilities file claims for single-dose drugs and biologicals. CMS made this happen by developing the JZ modifier “Zero drug amount discarded/not administered to any patient.”1 The CY 2023 Medicare Final Rule for both the Physician Fee Schedule and Hospital Outpatient Prospective Payment and Ambulatory Surgery Center (ASC) Payment Systems also includes the new modifier.2,3 It appears CMS is tying the new JZ modifier in with the long-standing JW modifier describing any discarded drug. Let’s review what you need to know.
DRUG REPORTING
In June 2016, CMS published Transmittal 3538 stipulating that, “Effective January 1, 2017, claims for discarded drug or biological amount not administered to any patient, shall be submitted using the JW modifier.”4 The JW modifier existed before 2017; however, CMS gave the Medicare Administrative Contractors (MACs) latitude to publish their own requirements. Since 2017, “CMS established a consistent policy among all MAC jurisdictions that required the use of the JW modifier for drugs separately payable under Medicare Part B with discarded amounts from single-dose containers.”1
“Discarded amounts” refers to the amount of discarded drug up to the amount indicated on the vial or package label. The discarded amount does not include any overfill from the manufacturer. For example, consider a vial of onabotulinumtoxinA (Botox, AbbVie) (J0585 Injection, onabotulinumtoxinA, 1 unit), labeled as 100 units. The surgeon administers 60 units and discards 40 units. When this happens, the claim should reflect two lines for the drug (J0585) (Figure 1).
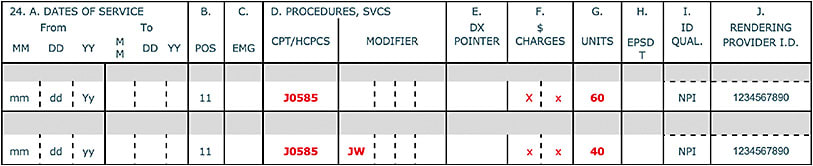
In contrast, do not use the JW modifier to identify discarded overfill beyond the package label. For example, the Eylea (aflibercept, Regeneron) label lists 2 mg/0.05 mL in a prefilled syringe. The volume beyond the 2 mg/0.05 mL represents overfill and does not belong on the claim.
Prior to 2023, claims for a single-use vial when the physician injected the full dose (eg, ranibizumab, [Lucentis, Genentech], Eylea, etc) did not require a modifier. However, on July 1, 2023, that changes. For example, any discarded or wasted drug from a single-dose prefilled syringe of Eylea (J0178) represents manufacturer overfill. Append the JZ modifier on the claim to identify “zero” units of Eylea was discarded/wasted (Figure 2). The JW modifier does not identify the discarded overfill.
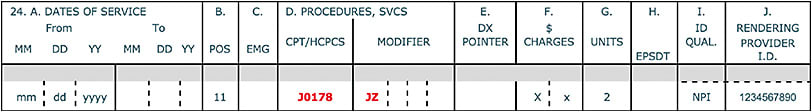
APPLICATION
In “buy and bill” scenarios where a physician or facility purchases the medication and files a claim to Medicare Part B for separately payable drugs, either a JW or JZ modifier is necessary. Do not use JW/JZ modifier with “white bagged” or specialty drugs that the provider or facility did not purchase.5 In the outpatient clinic space, retinal drugs (eg, Eylea, Lucentis, bevacizumab [Avastin, Genentech]) and Botox are the most frequent “buy and bill” drugs.
The same obligation to use JW or JZ also applies to ASC claims for separately reimbursed medications, such as Omidria (phenylephrine and ketorolac intraocular solution, Rayner) (J1097) and Dextenza (dexamethasone, Ocular Therapeutix) (J1096), which have a payment indicator of K2: “Drugs and biologicals paid separately when provided integral to a surgical procedure on ASC list; payment based on OPPS rate.”6
Use modifiers JW and JZ on claims for “not otherwise classified” (NOC) drug codes like J3490 and J3590. The FAQ published by CMS fails to address J7999 “Compounded drug, not otherwise classified.” Considering the comments about J3490 and J3590, it appears appropriate to use JW and JZ with J7999.
SUMMARY
Currently CMS requires the JW modifier. We have until July 1, 2023, before CMS requires claims without any discarded drug to have the JZ modifier. The effective date is July 1; however, CMS stipulates that “Claims that do not report the modifiers as appropriate on or after October 1, 2023, may be returned as unprocessable until claims are properly resubmitted.”1
As you can see from the addition of the JZ modifier, every claim to Medicare Part B for separately payable “buy and bill” drugs will have either one line with a JZ modifier or two lines — one with the injected units and the other with a JW modifier identifying the discarded units. OM
REFERENCES
- Discarded Drugs and Biologicals – JW Modifier and JZ Modifier Policy Frequently Asked Questions. CMS. https://www.cms.gov/medicare/medicare-fee-for-service-payment/hospitaloutpatientpps/downloads/jw-modifier-faqs.pdf . Accessed January 3, 2023.
- CY 2023 Medicare Physician Fee Schedule. CMS. https://www.federalregister.gov/documents/2022/11/18/2022-23873/medicare-and-medicaid-programs-cy-2023-payment-policies-under-the-physician-fee-schedule-and-other . Accessed January 3, 2023.
- CY 2023 Medicare Hospital Outpatient Prospective Payment and ASC Payment Systems. CMS. Published Nov. 23, 2022. https://www.federalregister.gov/documents/2022/11/23/2022-23918/medicare-program-hospital-outpatient-prospective-payment-and-ambulatory-surgical-center-payment . Accessed January 3, 2023.
- JW Modifier: Drug Amount discarded/not administered to any patient. Transmittal 3538. June 9, 2016. https://www.cms.gov/regulations-and-guidance/guidance/transmittals/downloads/r3538cp.pdf . Accessed January 3, 2023.
- CY 2023 Medicare Hospital Outpatient Prospective Payment and ASC Payment Systems. CMS. Published Nov. 18, 2022. https://www.federalregister.gov/documents/2022/11/18/2022-23873/medicare-and-medicaid-programs-cy-2023-payment-policies-under-the-physician-fee-schedule-and-other . Accessed January 3, 2023.
- Ambulatory Surgery Center Payment Indicators. Palmetto GBA. https://www.palmettogba.com/palmetto/rr.nsf/DIDC/59NDALJFK6~Specialties~Ambulatory%20Surgical%20Center . Accessed January 4, 2023.









