Despite the myriad new treatments that have become available for dry eye disease (DED) in recent decades, it continues to be a significant global health burden, with growing prevalence worldwide.1,2 In the United States alone, DED is estimated to affect approximately 37 million adults.3 Yet, despite these high numbers, fewer than half of the patients with DED have actually been diagnosed.2
As the number of patients who present with DED increases, so does the variation in patient demographics. Once considered a benign inconvenience that disproportionately affected aging women, evidence shows that the disease is on the rise among younger populations and those who work on digital devices.5 As we see these patients in our clinics, we have become increasingly aware of the physical and psychosocial burden of the disease, including pain and discomfort, fluctuating vision and a disruption in the completion of everyday activities like reading, working and driving.1
Most often, a patient’s symptoms lead them into an eye-care clinic, even if they are not sure of the root cause. In many cases, patients have tried to alleviate symptoms on their own or have visited multiple doctors without relief by the time they reach an ophthalmologist. It is important to recognize that DED is multifactorial and to consider all potential contributing factors and the root cause of the symptoms to determine the best treatment plan. Here’s my list of common contributors.
DIET AND NUTRITION LINKED TO INFLAMMATION
How they contribute
The modern diet has evolved substantially since our hunter-gatherer ancestors walked the earth. The average diet today has shifted to include more processed foods with higher levels of Omega-6 fatty acids as well as sugar, grains and meat, and fewer leafy vegetables, fruit and foods high in Omega-3 fatty acids. The result has been an increase in chronic inflammatory diseases worldwide.6 Diets high in Omega-6 are considered inflammatory, and evidence suggests that this type of diet can contribute to the inflammatory component of DED.7-9
Recommendation
Encourage lifestyle and diet modifications. Inform patients about the benefits of anti-inflammatory diets and nutritional supplements (ie, high-quality Omega-3 fatty acids).
DIGITAL EYE FATIGUE
Screen time increases
Several studies have cited the correlation between digital screen use and DED. Digital screen use is thought to impact blinking dynamics, specifically the quantity and completeness of blinks.10,11 Individuals who stare at screens for long durations tend to blink less often, thereby increasing ocular surface dryness. This was particularly apparent during the peak of the COVID-19 pandemic when individuals were working from home and socializing remotely using digital devices.
This increase in screen time was also observed in younger populations, as schools were conducted virtually, and as students became more reliant on games, smartphones and online-based activities. A 2018 study published by Gupta et al demonstrated a high level of mild meibomian gland atrophy in the pediatric population.12 A separate study in JAMA Pediatrics found that US-based children in the 12-to-13-year-old age group doubled their screen time to 7.7 hours per day in May 2020 compared to 3.8 hours per day before the pandemic.13 The data suggest that DED is a growing problem, largely because of trending habits and lifestyle in the digital era that we are living in.
Frequent breaks required
Stress the importance of taking breaks from screen use. I suggest the 20/20/20 rule — for every 20 minutes spent looking at a computer, smartphone or screen of any type, look at an object 20 feet away, blinking completely, for at least 20 seconds (also consider using preservative-free, lipid-containing artificial tears). This will give eyes a much-needed break and allow blink patterns and frequency to normalize.
OFTEN OVERLOOKED
Demodex blepharitis
Demodex blepharitis is a common lid margin disease that results from an infestation of Demodex mites. There are two types of Demodex mites: Demodex brevis and Demodex folliculorum. Demodex folliculorum feed off the oily sebum in eyelash follicles and leave behind waste and debris, known as collarettes, which appear as waxy buildup at the base of the lashes (Figure). Collarettes are the pathognomonic sign of Demodex blepharitis. While Demodex mites are largely ubiquitous — they are the most common ectoparasite found on humans — they can have a clinical impact if an infestation is present.
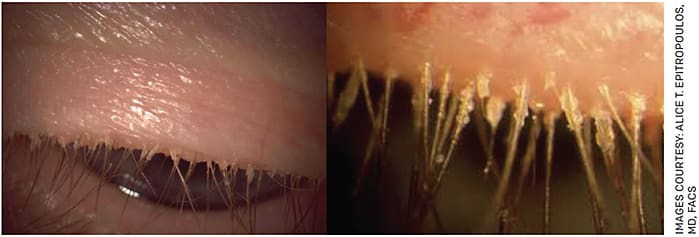
FIGURE. Left: Demodex blepharitis patient looking down during slit lamp exam with collarettes visible. Right: A close up view of collarettes on the same patient.
Diagnose and identify
Demodex blepharitis is often overlooked and, as a result, is commonly misdiagnosed or undiagnosed, especially since many of its symptoms — including dryness, tearing, burning, redness and blurry vision — are also symptoms of DED.14 Results from Trattler et al and the retrospective multi-center Titan study involving more than 1,000 patients revealed the presence of collarettes in 58% of patients visiting eye-care clinics for any reason and in 58.9% of dry eye patients.15,16 This is significant as it shows that, across diverse populations and geographies, more than half of all patients visiting ophthalmology or optometry practices could have Demodex blepharitis and that Demodex blepharitis may be an exacerbating or contributing factor to DED, if not the root cause.
Additionally, among those with DED who are receiving dry eye treatment (eg, lifitegrast, cyclosporine), 62% of the patients in the Titan study had Demodex blepharitis, suggesting that these drugs are not addressing the root cause of the problem. Demodex blepharitis is simple to diagnose and can be easily identified by examining the upper eyelid for collarettes when a patient looks down during a slit lamp exam. If this step in the eye exam is not performed, the disease can be missed, and often patients receive treatment and management options for other eye conditions with similar symptoms that are ineffective.
Help is on the way
No FDA-approved therapeutics are currently available for Demodex blepharitis, and existing management options are largely ineffective, inconvenient and result in poor patient compliance. Fortunately, a promising solution is in development. Tarsus Pharmaceuticals recently completed two pivotal studies collectively involving more than 800 patients that showed that its lead investigational therapeutic, TP-03 (lotilaner ophthalmic solution, 0.25%), met the primary and all the secondary endpoints and was well-tolerated. This therapeutic in development is designed to target the root cause of Demodex blepharitis — the Demodex mite itself — and has shown in clinical studies to eradicate mites, eliminate collarettes and resolve eyelid erythema in patients.16 In September, Tarsus submitted a new drug application to the FDA for TP-03 for the treatment of Demodex blepharitis.
Slit lamp advice
Have all patients look down during slit lamp exams to check for Demodex blepharitis, as it may be a contributing factor for DED. Alternatively, the patient may simply have Demodex blepharitis.
Don’t forget makeup
Improper application or removal of makeup may exacerbate DED or result in clogged meibomian gland orifices. Hypoallergenic brands usually have a lower likelihood of containing irritants that may worsen DED. Educate patients that makeup should be removed (using products that are oil and paraben-free) before bed. A gentle eyelid scrub can be applied after makeup removal to help prevent clogging meibomian glands. The use of eyelash extensions can result in blepharitis and dermatitis, especially since they can make lid hygiene more challenging.
Additionally, instruct patients to avoid sharing products or using saliva with makeup application and to replace makeup every 3-6 months.
DIAGNOSE SOONER, EDUCATE OFTEN
Combatting DED requires more than an awareness of lifestyle risk factors. Fortunately, as our knowledge of the disease has increased in recent years, so have the number of treatments designed to alleviate symptoms and reduce complications. The pipeline for innovative therapies and technologies is robust, which is good news for eye-care professionals and patients.
With novel therapies on the market and in development, it is important to stay ahead of DED to maintain proper eye health and prevent long-term consequences, such as the corneal surface damage that can lead to vision loss. To ensure optimal patient outcomes, take a comprehensive approach to identifying, diagnosing and managing the disease.
This means regularly screening all patients for DED. Questionnaires are quick and effective ways to initially assess a patient before they reach the exam room, to determine if additional testing is required.
Also, educate patients about the long-term risks of DED. Even if a patient is asymptomatic, explain the risks of the disease when/if left untreated and the importance of compliance to the recommended treatment.
IT TAKES A HOLISTIC APPROACH
DED is a growing health concern that can have serious physical, psychological and economic implications. Taking steps to identify and manage the disease earlier, as well as educating patients and providing resources on lifestyle modifications, may help reduce the associated severity of the disease. And remind patients that — as with any lifestyle change — results take time. OM
REFERENCES
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15:334–365.
- Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol. 2017;182:90-98.
- Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry Eye in the Beaver Dam offspring study: Prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799-806. iv. Projected US Census Data, 2015-2060. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf . Accessed May 10, 2022.
- Dana R, Bradley JL, Guerin A, et al. Estimated Prevalence and Incidence of Dry Eye Disease Based on Coding Analysis of a Large, All-age United States Health-Care System. Am J Ophthalmol. 2019;202:47-54.
- Blehm C, Vishnu S, Khattak A, Mitra S, Yee RW. Computer vision syndrome: a review. Surv Ophthalmol. 2005;50(3):253-262.
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365-379.
- Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77(6):937-946.
- Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S-1519S.
- Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of Oral Re-esterified Omega-3 Nutritional Supplementation on Dry Eyes. Cornea. 2016;35(9):1185-1191.
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334-365.
- LeBlanc AG, Gunnell KE, Prince SA, et al. The ubiquity of the screen: an overview of the risks and benefits of screen time in our modern world. Transl J Am Sports Med. 2017;2:104-113.
- Gupta PK, Stevens MN, Kashyap N, Priestley Y. Prevalence of Meibomian Gland Atrophy in a Pediatric Population. Cornea. 2018;37(4):426-430.
- Nagata JM, Cortez CA, Cattle CJ, et al. Screen Time Use Among US Adolescents During the COVID-19 Pandemic: Findings From the Adolescent Brain Cognitive Development (ABCD) Study. JAMA Pediatr. 2022;176(1):94-96.
- Basta-Juzbasić A, Subić JS, Ljubojević S. Demodex folliculorum in development of dermatitis rosaceiformis steroidica and rosacea-related diseases. Clin Dermatol. 2002;20(2):135-140.
- Trattler W, Karpecki P, Rapoport Y, et al. The Prevalence of Demodex Blepharitis in US Eye Care Clinic Patients as Determined by Collarettes: A Pathognomonic Sign. Clin Ophthalmol. 2022;16:1153-1164. Published 2022 Apr 15.
- TITAN study. 2021. Tarsus Pharmaceuticals, Inc. Data on file.









