Dry eye (DED) is a ubiquitous ophthalmic disease that will inevitably increase in incidence in the United States and worldwide as the population continues to age.1 A complex, multifactorial disease, dry eye commonly coexists with meibomian gland disease, allergic conjunctivitis, glaucoma and preservative hyper-sensitivity. Dry eye can also be induced or exacerbated by surgical interventions. As novel medications with vastly different mechanisms of action are developed to address nuanced forms of dry eye, practitioners will have more tools to address clinical signs and symptoms of dry eye in their patients.
Topical steroid therapy exemplified the very earliest efforts to abate inflammation, an important dry eye mechanism.2 This led to the approval of Eysuvis (loteprednol etabonate ophthalmic suspension, 0.25%, Kala Pharmaceuticals) in late 2020. With a novel mucus-penetrating particle vehicle, it was the first steroid specifically approved for episodic dry eye.3 A patient prone to glaucoma may best be served by either loteprednol, a selective ester steroid with significantly less proclivity to IOP elevations, or another class of agent altogether.
Another new-to-market medication, Regener-Eyes, is a preservative-free biological eyedrop that uses d-MAPPS (derived-Multiple Allogeneic Proteins Paracrine Signaling) technology to stimulate stem cell communication without cell contact. This option is usually prescribed for mild-to-moderate or severe DED, especially when there is corneal involvement such as punctate epithelial keratitis.
Recently, a variety of other novel medications, highlighted in part herein, have been developed to address additional factors implicated in DED.
TYRVAYA
Varenicline tartrate solution 0.03 mg (Tyrvaya, Oyster Point Pharma), approved in late 2021, is a novel dry eye treatment that avoids eyedrops altogether. Investigated through the MYSTIC Phase 2 randomized trial and the ONSET-2 Phase 3 randomized trial, Tyrvaya has repeatedly been shown to significantly improve signs and symptoms of dry eye.4,5 The primary endpoint in the MYSTIC trial was mean change from baseline in Schirmer’s score at day 84 and for ONSET-2 improvement in Schirmer’s test by 10 mm or more at week 4; however, tear production was shown to increase as quickly as 5 minutes after administration.4
While the exact mechanism of action is not completely understood, Tyrvaya is thought to be a neuro-stimulating agent, activating the parasympathetic pathway of the nasociliary branch of the trigeminal nerve in the nose, thereby increasing baseline tear production. The active ingredient in Tyrvaya, varenicline, has also been used as a smoking cessation aid. Given as an oral tablet in much greater systemic doses as Chantix, varenicline has an excellent tolerance. The side effect profile of Tyrvaya is relatively benign, with the most common adverse reaction being sneezing, which occurs in 82% of patients. Additional adverse reactions include cough, throat irritation and instillation-site (ie, nose) irritation.4,5
Given by nasal spray twice daily in each nostril, Tyrvaya can not only reduce treatment burden for patients, but is also an excellent option for patients who have difficulty with drops. For example, patients with ocular toxicity, reduced neck mobility or upper limb mobility, tremors, digital arthritis, who live alone or who struggle with self-administering eyedrops may all benefit from dry eye treatment via nasal spray.
In addition, Tyrvaya provides a solution to the challenging glaucoma patient on multiple medications to lower IOP or preserve filtration surgery, as they may not welcome another drop for what might be their secondary diagnosis of dry eye. Nasal delivery mitigates the compliance, toxicity and nuisance factors of burgeoning medicamentosa. Of note, Tyrvaya was not tested on patients who use CPAP, with prior sinus surgeries, with a history of PKP and those with recurrent nosebleeds.4 As such, no conclusions can be drawn about its effectiveness in these specific demographics.
Nevertheless, the approval of both a new delivery route and a novel mechanism of action in one new pharmaceutical portends subsequent exciting developments for dry eye patients.
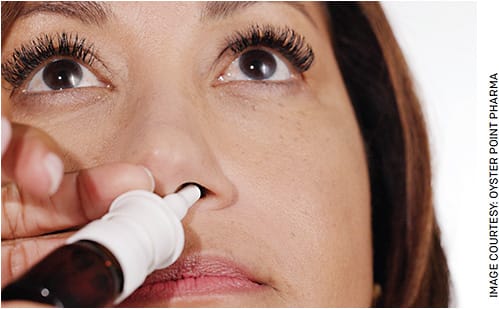
FIGURE. An example of correct Tyrvaya (Oyster Point Pharma) instillation.
REPROXALAP
Reproxalap (Aldeyra Therapeutics) is a new topical therapeutic currently being developed to address both allergic conjunctivitis and DED. Reproxalap is a novel, small-molecule immune-modulating covalent inhibitor of reactive aldehyde species (RASP).6,7
While the exact mechanism of action is not fully understood, RASP potentiates inflammation through a variety of inflammatory mediators and pathways. RASP inhibitors are the gatekeepers of inflammation. They work quickly and efficiently at the top of the inflammatory cascade before other drugs like steroids and immunomodulators that work downstream in the inflammatory process, before T cells become activated and before cytokines are released. As such, Reproxalap presumably reduces inflammation implicated in both allergic disease and dry eye as was demonstrated in Phase 2b and Phase 3 trials.
Phase 2b clinical trials demonstrated rapid, broad and clinically relevant symptomatic control, in conjunction with statistically significant improvement over vehicle in signs of DED as demonstrated by fluorescein staining, in DED patients over 12 weeks of therapy. In a Phase 2b trial, patients were randomized to reproxalap 0.1%, reproxalap 0.25% and placebo. Participants were then exposed to a controlled adverse environment, consisting of low humidity, for 90 minutes after a 12-week course of q.i.d. treatment. Relief for symptoms of dry eye was appreciated as early as 2 weeks into the treatment course, at the first follow-up visit.6 Patients experienced symptomatic improvement in a dose-dependent response, notably relative to grittiness and dryness.6 Researchers also appreciated improvements in nasal fluorescein staining in the 0.25% group.
For patients with allergic conjunctivitis, Reproxalap was evaluated in a randomized, double-blind Phase 2 trial and in the Phase 3 ALLEVIATE trial.7,8 In each of these studies, participants were randomized to various treatment groups, including reproxalap 0.25%, reproxalap 0.5% and vehicle. Participants who had been administered reproxalap noted improvement in symptoms including tearing, itching and redness.7,8 Higher doses of reproxalap (0.5% vs 0.25%) were associated with higher rates of redness after their first dose, suggestive of some instillation site reaction (ie, eye irritation).8 Aldyera selected a 0.25% regime to develop, given the clinical relevance observed with the improvement in ocular itching and conjunctival redness.7
A new drug application submission for reproxalap is anticipated later this year. This is a promising medication for those suffering from both dry eye and allergic conjunctivitis. Typically, muscarinic topical antihistamines dry the ocular surface, while topical therapies for dry eye and meibomianitis frequently create hypersensitivity or irritative effects on the allergic ocular surface. No class of agents other than steroids currently simultaneously serve these demographics, so a truly game-changing therapeutic for millions of patients with both conditions will be welcomed.
| Dry eye | MGD | Allergic conjunctivitis | Preservative hypersensitivity | Glaucoma | Corneal wound healing | Available now | |
| Loteprednol etabonate ophthalmic solution 0.25% | X | X | X | ||||
| Varenicline tartrate solution 0.03 mg | X | X (nasal instillation) |
X | ||||
| Reproxalap 0.25% | X | X | |||||
| Perfluorohexyloctane, 100% | X | X | X | ||||
| SkQ1 | X | X | X |
NOV03
In 2019, Bausch Health announced that it licensed Novaliq’s NOV03 investigational treatment for commercialization and development in the United States and Canada. NOV03 is a unique medication that has been demonstrated to address both DED and MGD. The active ingredient in NOV03, perfluorohexyloctane (F6H8), is a preservative-free ophthalmic solution that stabilizes the lipid component of the tear film and penetrates meibomian glands. Within the meibomian glands, the perfluorohexyloctane interacts with and assists with dissolving the viscous meibum in the glands.9
In the double-blind randomized Phase 2 saline-controlled trial SEECASE, NOV03 demonstrated improvement in both signs and symptoms of dry eye, specifically in total fluorescein staining, tear break-up time and in participant responses to the eye dryness score.10 These improvements can begin to be appreciated as early as 2 weeks into the full 8-week treatment. A low rate of instillation site reaction (ie, eye irritation) was reported in patients in a dose-dependent response, in 2.6% of patients receiving q.i.d. and 0.9% of those receiving b.i.d.10 Australia, New Zealand and Europe have approved equivalent ophthalmic drops, 100% perfluorohexyloctane, with an excellent safety profile.11 An additional consideration is that trials did not include patients on topical glaucoma medications, and, as such, interactions with additional topical ocular hypotensives have yet to be investigated.10,11
The semi-fluorinated alkane molecule class is also an outstanding water-free vehicle capable of carrying a wide variety of active pharmaceutical ingredients, including cyclosporine A 0.1% found in the Novaliq CyclASol preparation, now in a confirmatory ESSENCE II Phase 3 randomized controlled trial. In addition to water-free, preservative-free properties, the 20 µl drop size reduces drop overflow. The CyclASol clinical data shows tolerability similar to vehicle.12
VISOMITIN
Mitotech has been investigating a novel medication, Visomitin (SkQ1), a cardiolipin peroxidase inhibitor. Previously investigated in relation to senesce and neurodegenerative conditions,13 SkQ1 has demonstrated promising results relative to the ocular surface as well. SkQ1 has a novel mechanism of action as a mitochondrial-targeted antioxidant, addressing the oxidative stresses that occur in both DED and corneal wounds.14 As such, SkQ1 holds promise for dry eye that occurs postoperatively after cataract extraction.
Investigation into SkQ1 is ongoing. In the randomized, double-blind, placebo-controlled Phase 3 VISTA-2 trial, SkQ1 is being studied to treat moderate to severe DED.15 Primary endpoints in this study include improvement in central corneal staining, BCVA and eye discomfort.15 The tolerance of SkQ1 has been excellent, with a side-effect profile similar to that of an artificial tear.15 SkQ1 formulations do contain preservatives.
While the Phase 3 trial is ongoing, SkQ1 holds excellent potential for patients with dry eye exacerbated by such events as cataract surgery. Interestingly, mitochondrial antioxidants can also lower IOP.16 Commonly, glaucoma patients administering multiple vision-saving topical ocular hypotensive agents develop recalcitrant ocular surface disease. A dual mechanism controlling both dry eye and glaucoma would benefit a large, currently underserved patient base.
CONCLUSION
Dry eye is a complex, multifactorial disease that often coexists with other ocular and systemic pathology. Ongoing research has demonstrated great promise in the treatment of dry eye in new, innovative ways, while simultaneously addressing concomitant pathology. With this burgeoning research in the nuanced presentations of dry eye and related pathology, practitioners are now better able to assist patients in finding a solution that can provide relief. OM
REFERENCES
- Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol. 2017;182:90-98.
- Comstock TL, Sheppard JD. Loteprednol etabonate for inflammatory conditions of the anterior segment of the eye: twenty years of clinical experience with a retrometabolically designed corticosteroid. Expert Opin Pharmacother. 2018;19(4):337-353.
- Venkateswaran N, Bian Y, Gupta PK. Practical Guidance for the Use of Loteprednol Etabonate Ophthalmic Suspension 0.25% in the Management of Dry Eye Disease. Clin Ophthalmol. 2022;16:349-355. Published 2022 Feb 9.
- Wirta D, Vollmer P, Paauw J, et al. Efficacy and Safety of OC-01 (Varenicline Solution) Nasal Spray on Signs and Symptoms of Dry Eye Disease: The ONSET-2 Phase 3 Randomized Trial [published online ahead of print, 2021 Nov 10]. Ophthalmology. 2021;S0161-6420(21)00836-8.
- Quiroz-Mercado H, Hernandez-Quintela E, Chiu KH, Henry E, Nau JA. A phase II randomized trial to evaluate the long-term (12-week) efficacy and safety of OC-01 (varenicline solution) nasal spray for dry eye disease: The MYSTIC study [published online ahead of print, 2021 Dec 15]. Ocul Surf. 2021;24:15-21.
- Clark D, Tauber J, Sheppard J, Brady TC. Early Onset and Broad Activity of Reproxalap in a Randomized, Double-Masked, Vehicle-Controlled Phase 2b Trial in Dry Eye Disease. Am J Ophthalmol. 2021;226:22-31.
- Clark D, Karpecki P, Salapatek AM, Sheppard JD, Brady TC. Reproxalap Improves Signs and Symptoms of Allergic Conjunctivitis in an Allergen Chamber: A Real-World Model of Allergen Exposure. Clin Ophthalmol. 2022;16:15-23. Published 2022 Jan 4.
- Clark D, Cavanagh B, Shields AL, Karpecki P, Sheppard J, Brady TC. Clinically Relevant Activity of the Novel RASP Inhibitor Reproxalap in Allergic Conjunctivitis: The Phase 3 ALLEVIATE Trial. Am J Ophthalmol. 2021;230:60-67.
- Kroesser S, Spencer E, Grillenberger R, et al. Ocular and systemic distribution of 14C-perfluorohexyloctane following topical ocular administration to rabbits. Poster A0383 presented at: The Association for Research in Vision and Ophthalmology Annual Meeting 2018; Investigative Ophthalmology & Visual Science. 2018; 59, 2656.
- Tauber J, Wirta DL, Sall K, et al. A Randomized Clinical Study (SEECASE) to Assess Efficacy, Safety, and Tolerability of NOV03 for Treatment of Dry Eye Disease. Cornea. 2021;40(9):1132-1140.
- Son HS, Yildirim TM, Khoramnia R, Poompokawat P, Knorz MC, Auffarth GU. Semi-fluorinated Alkane Eye Drops Reduce Signs and Symptoms of Evaporative Dry Eye Disease After Cataract Surgery. J Refract Surg. 2020;36(7):474-480.
- Sheppard JD, Wirta DL, McLaurin E, Boehmer BE, Ciolino JB, Meides AS, Schlüter T, Ousler GW, Usner D, Krösser S. A Water-free 0.1% Cyclosporine A Solution for Treatment of Dry Eye Disease: Results of the Randomized Phase 2B/3 ESSENCE Study. Cornea. 2021;40(10):1290-1297.
- Shabalina IG, Vyssokikh MY, Gibanova N, et al. Improved health-span and lifespan in mtDNA mutator mice treated with the mitochondrially targeted antioxidant SkQ1. Aging (Albany NY). 2017;9(2):315-339.
- Zernii EY, Gancharova OS, Tiulina VV, Zamyatnin AA Jr, Philippov PP, Baksheeva VE, Senin II. Mitochondria-targeted antioxidant SKQ1 protects cornea from oxidative damage induced by ultraviolet irradiation and mechanical injury. BMC Ophthalmol. 2018;18(1):336. Published 2018 Dec 27.
- Mitotech and Essex Bio-Technology Complete Enrollment in VISTA-2 – a pivotal Phase 3 Clinical Study of SkQ1 for Dry Eye Disease. https://tinyurl.com/yrhmxt2i . Published August 25, 2020. Accessed February 14, 2022.
- Iomdina EN, Khoroshilova-Maslova IP, Robustova OV, Averina OA, Kovaleva NA, Aliev G, Reddy VP, Zamyatnin AA Jr, Skulachev MV, Senin II, Skulachev VP. Mitochondria-targeted antioxidant SkQ1 reverses glaucomatous lesions in rabbits. Front Biosci (Landmark Ed). 2015;20(5):892-901. Published 2015 Jan 1.










