TAKE-HOME POINTS
- Start by determining the source of the decreased vision: obstruction (corneal scarring) or distortion (irregular astigmatism).
- Scleral lenses can be quite effective at correcting vision for keratoconus patients.
- Consider corneal cross-linking (CXL) to limit keratoconus progression, maximize visual function and avoid surgical intervention as much as possible.
- Intracorneal ring segments can reshape the cornea and decrease the astigmatism, but they are invasive and carry risks.
- Combined PRK with CXL is in its infancy and requires more information from long-term studies before it can become commonplace.
Keratoconus is a progressive ectatic condition of the cornea that may progress from mild visual changes to severe loss of BCVA. The incidence had been estimated to be about 1 in 2,000, but more recent estimates are even higher.1-3 Keratoconus initially may present with gradually decreasing UCVA with increasing astigmatism. The irregularity of the thinning and ectasia may lead to higher order aberrations that are difficult to correct with traditional spectacles.1,4 Over time, as the condition progresses, rigid gas permeable (RGP) contact lenses and eventually corneal transplantation may be required.
Until corneal crosslinking (CXL) became available, there was no effective method to limit progression. Prior to CXL, it was estimated that 11% to 27% of keratoconus eventually progressed to having corneal transplant surgery.1,5,6 CXL, which was approved in the United States in 2016, acts by strengthening and stabilizing the corneal tissue and may significantly reduce the need for transplant. In the pivotal clinical trial used for approval, after 12 months, the treatment group was found to have an average flattening of maximum K value (Kmax) of 1.6 D, while the control group progressed by an average steepening of 1.0 D in Kmax. Thus, the difference was 2.6 D in 12 months.1,7
While CXL has been shown to be effective at slowing down progression, this does not necessarily coincide with visual rehabilitation. Therefore, eyecare professionals are still challenged with determining methods to improve vision and allow patients to be more functional. In this article, I will discuss my approach to rehabilitating vision for keratoconus patients.
LOOK TO THE SOURCE
The first step is to determine the source of the decreased vision. I consider keratoconus to affect vision in two ways: obstruction and distortion.
By obstruction, I am referring to corneal scarring that limits the ability for the image to pass through the cornea to allow for adequate vision. Often, if the central scarring is very dense, corneal transplant is needed. Scarring of this magnitude typically occurs in the later stage cones. Even with dense scarring, though, I have been pleasantly surprised at how well the vision may improve with other methods, and transplantation may be avoided. I will address this later in the article.
The second source of decreased vision is distortion, or irregular astigmatism. As keratoconus progresses, the cornea becomes steeper and more irregular. Higher order aberrations become more of an issue, and the patient may not be able to attain adequate vision with spectacles.
SCLERAL CONTACT LENSES
These patients are often treated with soft contact lenses initially followed by RGP contact lenses when the soft contact lenses are no longer sufficient. In my experience, scleral lenses may be quite effective at correcting vision. Almost any patient can be fitted for and obtain good vision with modern scleral lenses. In fact, in a paper by Baran et al looking at 89 candidate eyes in 59 patients, no cone was too steep to be fit and no eyes were excluded for severity of keratoconus.8 Failure to fit the eye is likely due to not using a lens with large enough diameter or one in which the fitter can customize the “vault” over the cone by adjusting the sagittal height.
In a paper by Deloss et al, 73 eyes were reviewed retrospectively. Thirty-seven eyes underwent keratoplasty, and 36 underwent scleral contact lens fittings — all 36 were fit successfully. Visual acuity was achieved more rapidly in the scleral cohort than the transplant cohort. The mean visual acuity was better with the scleral lens for all eyes (p<.0001) and when including only eyes with stage 4 ectasia (p<.001). In addition, a greater number of eyes achieved 20/25 after scleral lens than keratoplasty (p=.003).9
WHEN AND HOW TO ADDRESS PROGRESSION
While scleral lenses may be able to rehabilitate the vision in patients with even severe ectasia, they do not address progression. Therefore, regardless of visual acuity, limiting progression is always necessary to consider. Being that progression tends to slow down and even stop as patients age, it may not need to be addressed in an older patient if stability is confirmed. These patients may benefit from scleral lenses only along with monitoring for future progression.
For patients that are still progressing or at high risk for progression, attaining excellent vision with scleral lenses is not sufficient and the progression needs to be addressed. This is done with CXL.
From my perspective, the goal to managing these patients is to limit progression, maximize visual function and avoid surgical intervention as much as possible. While CXL is somewhat invasive, particularly when the epithelium is removed, and not without risks, its degree of invasiveness and risk is relatively small as compared with keratoplasty. Most patients are very functional within days of the procedure, and their eye is as strong as an untouched eye once the epithelium heals. While a post-keratoplasty patient may attain excellent vision, they are also subject to a lifetime of maintenance and risk following surgery. These risks include infection, rejection, cataracts, glaucoma and trauma. Therefore, a 20-year-old patient with 20/20 vision after keratoplasty may not be considered a finished product and complete success.
I have dealt with this many times. One patient underwent transplant surgery at age 18 prior to CXL being available. He attained 20/20 vision fairly rapidly after surgery. Much like many teenagers, though, he was not able to avoid certain rough physical activities and eventually ruptured his wound. We repaired the wound and he again attained 20/20 vision. Unfortunately, he was non-compliant with his medications and lost to follow up. He eventually returned with graft rejection. Again, we successfully treated this, and his excellent vision returned. As this case demonstrates, an early successful outcome from keratoplasty may not mean complete success. This patient will require a lifetime of close follow up and maintenance.
This case teaches us many lessons. Because of the lifetime risks of transplant, any time I can avoid surgery, I try to do so. The notion that a patient may now be subjected to long-term contact lens wear by only trying crosslinking and scleral lenses is not a sufficient reason to me to jump to keratoplasty, as a high number of transplant patients also end up needing contact lenses as well. As concluded in the Deloss paper, any patient with keratoconus should receive a scleral lens evaluation prior to proceeding with keratoplasty.9 In my experience, a history of contact lens intolerance or inability to fit contact lenses previously does not deter me in most cases. I have found that while many patients may be intolerant to contact lenses prior to crosslinking, the shape modification that is attained with crosslinking makes them more tolerant to the lenses. Also, with the contact lens modifications listed above, almost any cornea can be fit, regardless of steepness. Even in patients with what appears to be a visually limiting central scar, I have been surprised by how much visual improvement they attain with a scleral lens, and keratoplasty is often avoided.
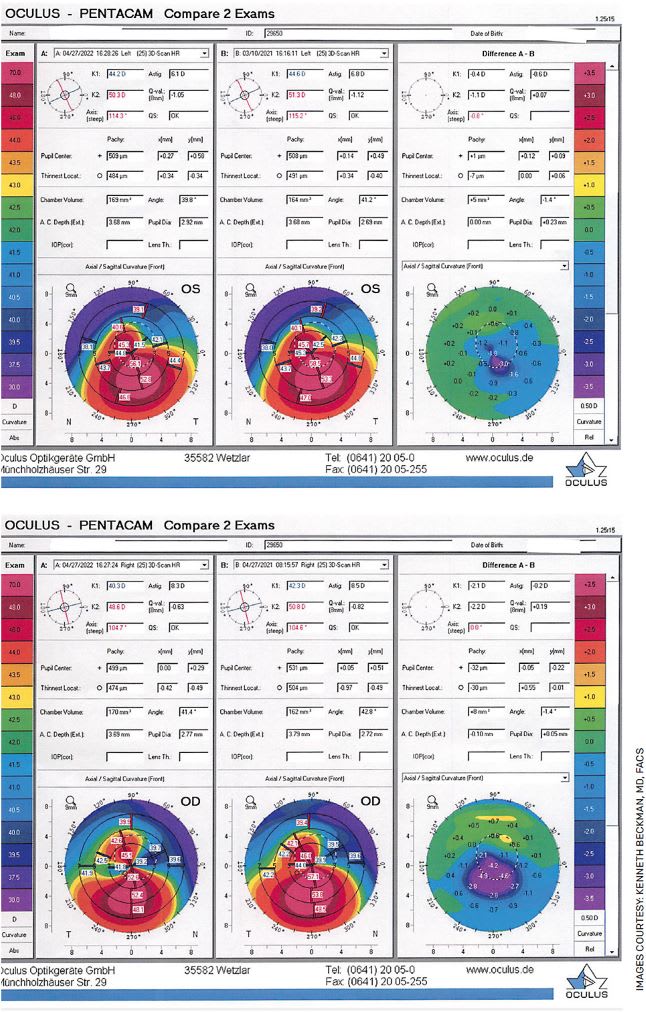
FIGURE. A 25-year-old female with moderate keratoconus who underwent corneal crosslinking (CXL) in each eye approximately 1 year ago. Her pre-treatment Kmax in her left eye was 59.5 and in her right eye was 57.8. Her post-treatment Kmax in her left eye was 56.4 and her right eye was 53.4 at 1 year. The images were the difference map for each eye (top, left; bottom, right). The left column is post-treatment, and the center column is pre-treatment. The right column is the difference map, which shows an island of blue that represents flattening in the area that was the steepest. This is an excellent example of the rapid and dramatic improvement in corneal shape that we often see with CXL.
OTHER REHABILITATION OPTIONS
CXL plays a role
A number of my crosslinking patients have developed so much remodeling that they do not even need scleral lenses after treatment. Some of them are able to achieve excellent vision with spectacles after treatment, or even uncorrected entirely, when they were not able to do so previously.
I always explain to patients prior to treatment that, unlike LASIK, the treatment is about stabilizing the cornea rather than correcting the refractive error. Still, many patients achieve significant improvement.
Being that this is about stabilization and not recovery, it is imperative that we catch and treat these patients early before they suffer permanent vision loss.
Intracorneal ring segments
These ring segments are placed within the corneal stroma and are used to reshape the cornea and decrease the astigmatism. By making the cornea more spherical, the patient may attain better vision with or without correction. The patient may also be more easily fit with contact lenses.
That being said, this is an invasive procedure with risks such as infection and erosion of the ring segments. In addition, this procedure does not slow progression. Prior to the availability of CXL, intracorneal ring segments may have been used to allow the patient to attain the best vision possible until they inevitably progressed to a point that surgery may have been needed.
With the advent of CXL, as well as the advances in scleral lenses, this procedure is not used very often. Some have advocated using these segments at the time of crosslinking, but I prefer to allow crosslinking to alter the shape of the cornea on its own and see where the patient lands.
While I have done CXL on several patients with ring segments in place (because the segments did not stop progression), I do not remove the segments prior to proceeding. I have not found the need to place these segments in my patients for the most part because of the success of scleral lenses.
For a patient who has been crosslinked and is truly unable to wear scleral lenses, this may be a nice addition to help them attain their best possible vision.
Laser refractive surgery
Finally, there is much interest in laser refractive surgery for these patients. While LASIK in keratoconus carries the risks of ectasia, photorefractive keratectomy (PRK) may not have the same risks, particularly when done in conjunction with or after crosslinking. The theory that CXL will merely stabilize the cornea and not correct refractive error has led to the need to address the residual refractive error with PRK. Kymionis et al evaluated a series of 10 eyes that underwent simultaneous sequential PRK followed by CXL and were followed for approximately 5 years. Nine of 10 eyes reached a spherical equivalent refractive error of +/-0.50 D, and all 10 were within +/-1.0 D. None lost a single line of corrected visual acuity, and one gained one line. All demonstrated stability during the 5-year period.10
I have seen so much remodeling with crosslinking alone that I prefer to have PRK done after the crosslinking has a chance to work rather than doing so at the same time. One concern about doing the crosslinking first and the PRK later is that we would be removing crosslinked tissue with the PRK and potentially losing some of the stabilization. By doing the PRK first and then crosslinking at the same sitting, we are able to remove tissue then crosslink the remaining tissue for added stability, which seems ideal. Of course, it is then difficult to account for the refractive error changes that would be attained from CXL alone, and there is the risk for over flattening and a significant hyperopic response. This strategy is still in its infancy, and I prefer to wait until more information is available before trying it routinely.
SUMMARY
Treatment for keratoconus has improved dramatically in recent years. We now have an excellent treatment available to limit progression in CXL. We also have an excellent non-invasive way to rehabilitate the vision in these patients with scleral lenses. Being that these can be fit on almost any patient and excellent vision may be attained in almost any patient other than those with a central, vision limited scar, the use of CXL and scleral lenses has become my primary method for rehabilitating vision in keratoconus. OM
REFERENCES
- Beckman KA, Gupta PK, Farid M, et al. Corneal crosslinking: Current protocols and clinical approach. J Cataract Refract Surg. 2019;45(11):1670-1679.
- Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33(4):157-205.
- Ferdi AC, Nguyen V, Gore DM, Allan BD, Rozema JJ, Watson SL. Keratoconus Natural Progression: A Systematic Review and Meta-analysis of 11,529 Eyes. Ophthalmology. 2019;126(7):935-945.
- Nakagawa T, Maeda N, Kosaki R, et al. Higher-order aberrations due to the posterior corneal surface in patients with keratoconus. Invest Ophthalmol Vis Sci. 2009;50(6):2660-2665.
- Javadi MA, Motlagh BF, Jafarinasab MR, et al. Outcomes of penetrating keratoplasty in keratoconus. Cornea. 2005;24(8):941-946.
- Mamalis N, Anderson CW, Kreisler KR, Lundergan MK, Olson RJ. Changing trends in the indications for penetrating keratoplasty. Arch Ophthalmol. 1992;110(10):1409-1411.
- Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK; United States Crosslinking Study Group. United States Multicenter Clinical Trial of Corneal Collagen Crosslinking for Keratoconus Treatment [published correction appears in Ophthalmology. 2017 Dec;124(12 ):1878]. Ophthalmology. 2017;124(9):1259-1270.
- Baran I, Bradley JA, Alipour F, Rosenthal P, Le HG, Jacobs DS. PROSE treatment of corneal ectasia. Cont Lens Anterior Eye. 2012;35(5):222-227.
- Deloss KS, Fatteh NH, Hood CT. Prosthetic replacement of the ocular surface ecosystem (PROSE) scleral device compared to keratoplasty for the treatment of corneal ectasia. Am J Ophthalmol 2014:974-982.
- Kymionis G, Kontadakis G, Grentzelos M, Petrelli M. Long-Term Follow-Up of Combined Photorefractive Keratectomy and Corneal Crosslinking in Keratoconus Suspects. Clin Ophthalmol. 2021;15:2403-2410. Published 2021 Jun 9.









