Have you ever seen a persistent epithelial defect that would not heal despite all your typical treatments, such as bandage contact lens and aggressive lubrication? Were you worried that a secondary infectious keratitis did or would occur if the epithelium didn’t close? Were you surprised that the patient had little to no pain despite a large epithelial defect?
If this sounds familiar (and maybe brings back some bad memories), you have probably experienced the frustration of treating neurotrophic keratitis (NK). NK is a degenerative condition of the cornea in which a denervation of the trigeminal nerve results in a decrease or loss of corneal sensitivity associated with poor corneal epithelial healing. A lack of corneal sensation leads to alteration in epithelial homeostasis as well as decreased blinking and tear production.
Thankfully, we now have an FDA-approved drug to treat NK: cenegermin-bkbj (Oxervate, Dompé). Previously, we struggled with existing therapies aimed at encouraging epithelialization of the cornea by indirect means without any medical therapy available to regenerate corneal nerves.
In this article, I will discuss the three stages of NK, its frequent causes and how to incorporate Oxervate into your practice.
STAGES OF NEUROTROPHIC KERATOPATHY
Stage 1
Mild stage 1 NK manifests as punctate epithelial keratopathy, which is easily mistaken as simple dry eye-related punctate keratitis. Pay attention to those patients with unilateral punctate keratopathy that is out of proportion to their dry eye symptoms. Treat this as you would with your typical dry eye protocol, and you may get some improvement, but not always resolution (Figure 1).
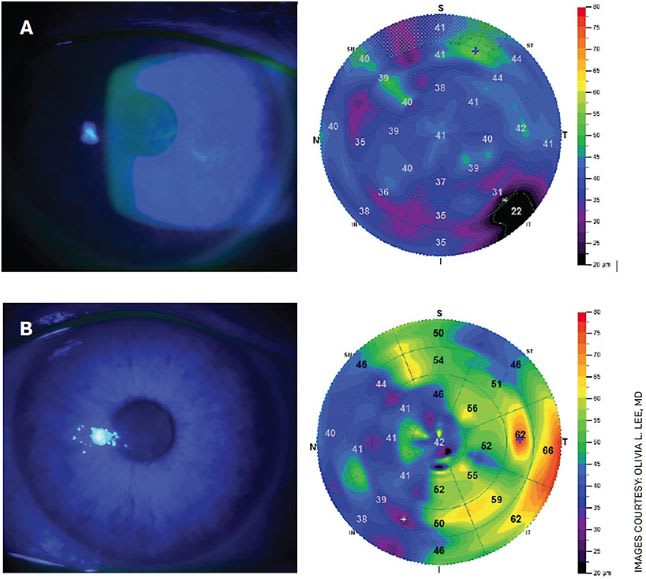
Figure 1A. Slit lamp photos and epithelial thickness maps in Stage 1 NK secondary to prior herpes simplex epithelial keratitis before (top left) and after (top right) Oxervate therapy.Figure 1B. Slit lamp photos and confocal microscopy in Stage 2 NK in trigeminal neuralgia before (bottom left) and after (bottom right) Oxervate treatment.
Stage 2
Stage 2 NK is characterized by persistent epithelial defect of the cornea. It may or may not resolve with measures such as bandage contact lens, amniotic membrane, tarsorrhaphy and moisture chamber. These cases can be tough when you have tried everything and cannot get the epithelial defect to completely resolve. Or it may resolve temporarily only to recur later (Figure 2).
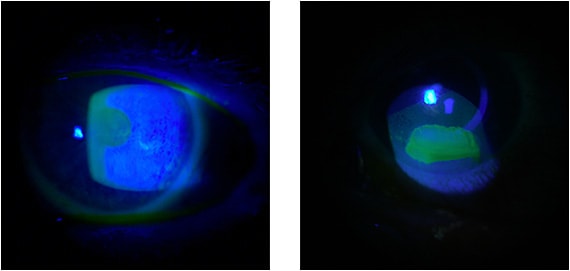
Figure 2. Slit lamp photos and confocal microscopy in Stage 2 NK in trigeminal neuralgia before (left) and after (right) Oxervate treatment.
Stage 3
The most severe form of NK is stage 3 disease, which is the result of prolonged epithelial breakdown, exposing the underlying stroma to enzymatic degradation and stromal thinning. Ultimately, this may progress with severe consequences such as secondary infection, neovascularization, opacification, melting and perforation.
NK CAUSES
The most frequent causes of NK are neurosurgical intervention resulting in trigeminal nerve ablation, tumors or stroke affecting the trigeminal nerve, herpetic eye disease (both zoster and simplex), long-term use of contact lenses, chronic use of topical eye medications such as beta blockers and NSAIDs, corneal incisional surgery or trauma, chemical eye burn and systemic diseases (eg, diabetes mellitus, multiple sclerosis and congenital syndromes such as Riley-Day syndrome).1
When you suspect the diagnosis of NK, remember to check the corneal sensation prior to instilling any topical anesthetics. You may lose your chance to accurately check the corneal sensation if your technician checks the IOP with anesthetic before you come into the room. Instead of waiting for the anesthetic to wear off, it would be preferable to instruct your staff not to apply any drops until after the sensation is checked. The best way to check corneal sensation is with a Cochet-Bonnet aesthesiometer (Luneau Technology), which will give you both qualitative and quantitative information. Alternatively, you may use a cotton wisp from the tip of a sterile cotton swab or a piece of unminted dental floss. Test both eyes in all four quadrants, and record the findings as normal, reduced or absent relative to the unaffected eye.
OXERVATE
About Oxervate
Oxervate is a recombinant human nerve growth factor (rhNGF) eyedrop formulation approved by the FDA for the treatment of NK. The drug is applied topically six times per day for 8 weeks. Cenegermin was evaluated in two randomized placebo-controlled studies3,4 involving patients with moderate or severe NK. It demonstrated efficacy in inducing complete corneal healing and reducing disease progression in 78% at 8 weeks, as well as a low recurrence rate of 4% after the treatment.
The most common side effect is mild ocular pain, which is generally tolerable and subsides after conclusion of therapy. I tell patients it is a good sign that they feel pain because it means that corneal innervation is being restored (Figure 3).
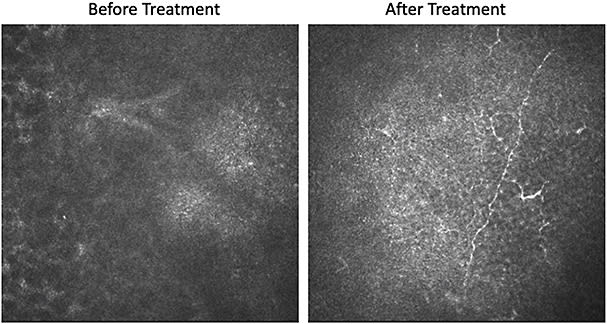
Figure 3. In vivo confocal microscopy demonstrating nerve regrowth before (left) and after (right) 8 weeks of Oxervate therapy.
When to consider treatment
Once the diagnosis of corneal hypoesthesia is established and you have reason to believe it is responsible for corneal manifestations on exam consistent with NK, consider treatment with Oxervate. This is especially true if you have already tried multiple treatments but the disease is recalcitrant or recurrent. The problem may lie in that the root cause of keratitis (ie, corneal anesthesia) has not been addressed. The only other treatment that can address this is corneal neurotization surgery, which is not offered at many centers.2
I suggest prescribing Oxervate on a stage 2 NK with persistent epithelial defect or a stage 1 NK with visually significant epitheliopathy.
The ordering process
A physician can order the drug through Dompé by completing the Oxervate Patient Enrollment Form found on the resource portal of the Oxervate website.
Your office may need to assist with prior authorization from the patient’s insurance; a sample appeals letter can also be found on the Oxervate website. The Dompé Connect to Care team takes it from there, including patient education on instructions of use. The drug is shipped directly to the patient and must be refrigerated upon receipt.
Some of my patients have expressed concern about the cost of the medication, but the patient assistance program has been a wonderful resource to make the medication affordable or no cost at all. The patient enrollment form gives the Dompé Connect to Care team the patient’s contact and insurance information. After the prior authorization is approved, if the copay is too high, the patient can elect to use the patient assistance program. They take care of it, and the patients seem very happy about the reduction in cost.
CONCLUSION
There is no reason not to try the standard treatments you have already being using for NK and add Oxervate to your armamentarium. Practically speaking, the time between submission of the enrollment form and the patient receiving the drug on their doorstep is a couple of weeks. You can use that time to employ all the things you would have done otherwise — cover with topical antibiotics, aggressive lubrication, lid tape, amniotic membrane, etc. Improving the ocular surface environment and making it conducive for epithelial healing with other treatments is an additive to the neuro-regenerative treatment properties of Oxervate.
Furthermore, do not wait until you are dealing with an impending perforation to order Oxervate, expecting it to arrive the next day. Before you spend time desperately exhausting all other options, think about Oxervate. This drug is a powerful tool in your toolbox, potentially the only one that can restore the function of corneal nerves in NK patients. OM
REFERENCES
- Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571-579. Published 2014 Mar 19.
- Fung SSM, Catapano J, Elbaz U, Zuker RM, Borschel GH, Ali A. In Vivo Confocal Microscopy Reveals Corneal Reinnervation After Treatment of Neurotrophic Keratopathy With Corneal Neurotization. Cornea. 2018;37(1):109-112.
- Bonini S, Lambiase A, Rama P, et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018;125(9):1332-1343.
- Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology. 2020;127(1):14-26.









