Intraocular injections are a keystone in the management of numerous retinal conditions. Typically, these injections deliver drug to the retina via a vitreous depot. However, suprachoroidal injections offer a more targeted alternative, achieving chorioretinal concentrations 10 times greater than that of typical intravitreal injections as well as reduced concentrations in the anterior segment.1,2 Thus, drug delivery via the suprachoroidal space (SCS) has significant potential advantages in the treatment of posterior segment diseases.
Here, we discuss the available and pipeline treatments delivered via the SCS.
CLS-TA (XIPERE)
In October 2021, triamcinolone acetonide injectable suspension (Xipere; Bausch + Lomb and Clearside Biomedical) for suprachoroidal use (CLS-TA) received FDA approval for use in the treatment of macular edema associated with uveitis.
PEACHTREE, one of the pivotal trials leading to its approval, was comprised of 160 patients randomized 3:2 to CLS-TA vs sham, administered at day 0 and week 12. It was the first Phase 3 trial for this indication with a primary endpoint of visual acuity gain: the proportion of patients gaining 15 or more letters.3 At week 24, 47% of subjects in the CLS-TA arm gained 15 or more letters compared to 16% in the control arm (p < 0.001).3 Mean reduction in central subfield thickness (CST) was 153 µm vs 18 µm in favor of the CLS-TA group (p < 0.001).3 Also, elevated IOP was seen in 11.5% of the CLS-TA patients compared to 15.6% of the control group.3
The time to rescue was also evaluated over an additional 24-week period in the extension study, MAGNOLIA.4 Over the total 48-week period studied in both trials, the median time to rescue was 257 days in the CLS-TA group compared to 55.5 days in the sham group.4 CLS-TA patients who did not require rescue over the year-long period continued to show robust treatment response, with a gain of 12 letters and anatomic improvement of 175 µm at week 48.4 As a result, the FDA approved CLS-TA in October 2021 for the treatment of uveitic macular edema. Xipere is being rolled out in the United States via physician trainers to retina and uveitis specialists, each of whom are having virtual training to master the process of injecting in the SCS.
With the advent of CLS-TA demonstrating the feasibility of safely delivering the steroid to the SCS with long-lasting therapeutic effect, interest arose in delivery to the SCS for other agents.
OTHER SCS MEDICINES BEING EXPLORED
Tyrosine kinase inhibitors (TKI)
TKIs are another approach at broader VEGF inhibition. While current agents affect the VEGF cascade by binding to VEGF-A in the extracellular space, preventing it from binding to the receptors on cell membranes, TKIs target a different part of the same pathway. TKIs inhibit the intracellular signaling of the VEGF receptors, effectively making the receptors dormant and non-responsive to extracellular stimuli.
CLS-AX (axitinib injectable suspension, Clearside Biomedical) is one such TKI that inhibits VEGF receptors 1, 2 and 3, resulting in a broad blockade of the VEGF cascade. Early pre-clinical studies have shown potential. CLS-AX decreases retinal and choroidal neovascularization in vitro better than anti-VEGF-A or anti-PDGF-B, alone or combined,5 and prevents the onset of neovascularization better than equivalent doses of other TKIs in a murine ocular burn model.6 Animal models have also confirmed a biologic signal, resulting in reduced retinal and choroidal neovascularization in rodents dosed systemically5 and pigs dosed via the SCS.7
A Phase 1/2a, dose-escalating trial of CLS-AX — SCS-delivered axitinib injectable suspension for the treatment of AMD — is currently enrolling.8 Cohorts 1 and 2, consisting of 11 patients who had received a mean of 9.1 intravitreal anti-VEGF injections in the 12 months before enrollment, tolerated CLS-AX well. There were no adverse events related to CLS-AX or dose-limiting toxicities.8 Both visual acuity and CST remained stable at 3 months post CLS-AX, with 55% of patients being retreated with anti-VEGF at 2 months and 36% going at least 3 months prior to retreatment.8
These data supported escalating the dose further by advancing to Cohort 3.8
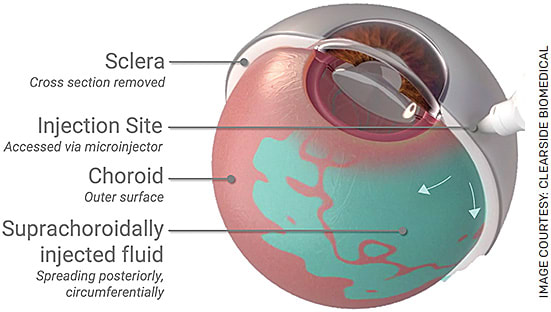
FIGURE. Suprachoroidal injection via a microneedle. Fluid enters the suprachoroidal space, the potential space between the choroid and sclera.
Gene therapy
Given the high treatment burden for patients with exudative retinal pathologies, the use of gene therapy to elicit extended therapeutic response has become an attractive target and the subject of numerous trials. Conditions include AMD, diabetic macular edema (DME) and diabetic retinopathy (DR).9-11 Strategies for the delivery of gene therapy include standard intravitreal injection, surgical subretinal injection and suprachoroidal injection.
ADVM-022 (Adverum Biotechnologies) encodes aflibercept and is optimized for the intravitreal approach, with improved penetration of the internal limiting membrane and retinal transduction.12 Phase 2 trials have resulted in >80% reduction in annualized anti-VEGF retreatment in patients with neovascular AMD;9 however, this has also resulted in significant intraocular inflammation, hypotony and dose-limiting toxicity with higher doses in a DME population.10
Surgical subretinal administration of RGX-314 (Regenxbio), encoding an anti-VEGF antibody fragment via an AAV8 vector that is more efficient at gene delivery to photoreceptors than AAV2,13 resulted in stable visual acuity and CST over a 2-year span in later cohorts, with an approximate 60% to 80% reduction in retreatments.11 Although no instances of immune response or drug-related inflammation were seen,11 this remains a surgical approach with all the inherent disadvantages and risks of vitrectomy.
An advantage of administering gene therapy via the SCS is the non-surgical approach with the potential for transducing near the target tissue and possibly mitigating inflammation.
Two Phase 2 trials of SCS-delivered RGX-314 are currently under way: one for AMD and another for DR.11,14 The first two AMD cohorts demonstrated stable vision and anatomy, with >70% reduction in annualized retreatments by month 6. While intraocular inflammation was seen in 23% of patients, they were all mild, resolving within days to weeks on topical steroids.11 Of the first cohort of DR patients, 33% had a two-step improvement of their Diabetic Retinopathy Severity Scale scores at month 3 and 47% at month 6,14 with only one case of mild episcleritis (1/15) that resolved with topical steroids.14
If higher-dose cohorts continue to show lasting benefit and similar safety outcomes with suprachoroidal gene therapy, managing the occasional episode of mild inflammation may well make this in-office delivery route preferable to either the intravitreal or subretinal approach.
Viral-like particle bioconjugates
Although radiation, the gold standard for the treatment of choroidal melanomas, is highly effective, it has many deleterious side effects, including vision loss.15 An alternative under development, AU-011 (Aura Biosciences), is a viral-like particle bioconjugate (VPB), a first-in-class nanoparticle agent derived from the human papillomavirus (HPV), taking advantage of HPV’s ability to preferentially bind to tumor cells,16 and conjugated with infrared-activated particles.17 Once AU-011 has bound to the tumor, it is light-activated with a 689-nm laser, leading to tumor cell necrosis.18,19
A Phase 1b/2 open-label, ascending dose trial with intravitreal AU-011 achieved a tumor control rate of 64% and vision preservation (loss of <15 letters) of 71% in patients with small tumors with active growth receiving two cycles of treatment.18 However, the rates of vitritis and anterior chamber (AC) inflammation were 91% and 71.5%, respectively.18 This is in contrast to early results from a Phase 2 trial using suprachoroidal AU-011, yielding no significant vitritis and 23% with AC inflammation.19
Though results are early, with more cohorts to follow, this favorable safety profile of the suprachoroidal approach may help to improve visual outcomes over intravitreal administration.
CONCLUSION
With the advent of Xipere, suprachoroidal drug delivery has become a clinical reality.
Offering potential advantages of posterior targeting, SCS-based therapies may help to advance our treatment of varied chorioretinal pathologies — from uveitic macular edema and wet AMD — to DR and choroidal melanoma. As such, the suprachoroidal approach is currently the focus of considerable research interest. OM
REFERENCES
- Patel SR, Lin AS, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res. 2011;28:166-176.
- Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53(8):4433-4441. Published 2012 Jul 1.
- Yeh S, Khurana RN, Shah M, et al. Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: Phase 3 Randomized Trial. Ophthalmology. 2020;127(7):948-955.
- Khurana RN, Merrill P, Yeh S, et al. Extension study of the safety and efficacy of CLS-TA for treatment of macular oedema associated with non-infectious uveitis (MAGNOLIA) [published online ahead of print, 2021 Mar 12]. Br J Ophthalmol. 2021;bjophthalmol-2020-317560.
- Giddabasappa A, Lalwani K, Norberg R, et al. Axitinib inhibits retinal and choroidal neovascularization in in vitro and in vivo models. Exp Eye Res. 2016;145:373-379.
- Yuan X, Marcano DC, Shin CS, et al. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. 2015;9:1749-1758.
- Bhisitkul R, Kansara V, Ciulla TA. Suprachoroidal CLS-AX (axitinib injectable suspension) as a potential long-acting therapy for neovascular agerelated macular degeneration (nAMD). Presented at: American Academy of Ophthalmology virtual annual meeting; November 2020.
- Clearside Biomedical Announces Positive Safety Results from OASIS Phase 1/2a Clinical Trial of CLS-AX (axitinib injectable suspension) for the Treatment of Wet AMD. Press release. 2021. https://www.globenewswire.com/news-release/2021/12/21/2355973/0/en/Clearside-Biomedical-Announces-Positive-Safety-Results-from-OASIS-Phase-1-2a-Clinical-Trial-of-CLS-AX-axitinib-injectable-suspension-for-the-Treatment-of-Wet-AMD.html
- Pieramici D. Intravitreal Gene Therapy for Neovascular AMD - Phase 1 OPTIC Study. Presented during the ASRS 2021 annual meeting; San Antonio, TX.
- Boyer D. Intravitreal Gene Therapy for Diabetic Macular Edema with ADVM-022: Prospective, Randomized Phase 2 INFINITY Trial. Paper presented at: American Academy of Ophthalmology; 2021; New Orleans.
- Avery R. Two Year Results from the Subretinal RGX-314 Gene Therapy Phase 1/2a Study for the Treatment of Neovascular AMD, and an Update on Suprachoroidal Trials. American Academy of Ophthalmology Subspecialty Day; 2021, 2021; New Orleans.
- Dalkara D, Byrne LC, Klimczak RR, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5(189):189ra76.
- Vandenberghe LH, Bell P, Maguire AM, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey [published correction appears in Sci Transl Med. 2011 Dec 7;3(112):112er9]. Sci Transl Med. 2011;3(88):88ra54.
- Klufas M. Suprachoroidal Delivery of RGX-314 for Diabetic Retinopathy Without CI-DME: Results from the Phase II ALTITUDETM Study. Angiogenesis, Exudation and Degeneration; 2022, 2022; Virtual.
- Aziz HA, Singh N, Bena J, Wilkinson A, Singh AD. Vision Loss Following Episcleral Brachytherapy for Uveal Melanoma: Development of a Vision Prognostication Tool. JAMA Ophthalmol. 2016;134(6):615-620.
- Kines RC, Cerio RJ, Roberts JN, et al. Human papillomavirus capsids preferentially bind and infect tumor cells. Int J Cancer. 2016;138:901-911.
- Aura Biosciences. Choroidal Melanoma. https://aurabiosciences.com/pipeline-programs/choroidal-melanoma/ . Accessed June 8, 2022.
- Shields CL. A Phase 1b/2 Trial of AU-011, an Investigational, Virus-Like Drug Conjugate (VDC) for the Treatment of Primary Indeterminate Lesions and Small Choroidal Melanoma (IL/CM) using Intravitreal Administration. Presented at: American Academy of Ophthalmology virtual annual meeting; 2021; New Orleans.
- Demirci H. A Phase 2 Trial of AU-011, an Investigational, Virus-Like Drug Conjugate (VDC) for the Treatment of Primary Indeterminate Lesions and Small Choroidal Melanoma (IL/CM) using Suprachoroidal Administration. Presented at: American Academy of Ophthalmology virtual annual meeting; 2021; New Orleans.









