In January 2022, the FDA granted the retina community — and millions of patients — the newest approved intravitreal treatment, faricimab (Vabysmo, Genentech), for both wet AMD and diabetic macular edema (DME). Two landmark Phase 3 trials, TENAYA/LUCERNE in patients with wet AMD and YOSEMITE/RHINE in patients with DME, showed that Vabysmo is safe, durable and effective in treating these disease states.
Vabysmo enters the market at a time when retina specialists are as busy as prior to the pandemic, if not more so. Its MOA is one of dual pathway inhibition of both VEGF-A and Ang-2 as a bispecific antibody.1,2 This is important because in pathological vessels, upregulation of Ang-2 leads to vascular leakage, inflammation and neovascularization.3-5 Pre-clinical data supports this, for dual inhibition of both Ang-2 and VEGF-A demonstrated increased vascular stability compared to VEGF-A inhibition alone.6
While current medications are safe and effective, treatment burden for both the doctor and the patient is real. A retrospective study of 49,485 patients on anti-VEGF monotherapy for wet AMD demonstrated a linear relationship between vision and number of injections over the first year of treatment, with seven injections showing just one letter of gain.7 Real-world studies corroborate these findings. Wet AMD patients receive, on average, 6.0-7.6 injections over the first year of treatment, yielding just 0.6-1.1 letter of vision gain.8,9
The story is more of the same for DME patients, but with slightly more letters gained. A retrospective study of 28,658 patients receiving anti-VEGF monotherapy reported a mean of 6.4 injections and a gain of 4.2 letters over a one-year timeframe.10 These are notably lower vision gains than those reported from the controlled clinical trials where patients are examined at retina study site clinics every month.
Furthermore, the ASRS Preferences and Trends Survey 2020 stated that the greatest unmet needs for treatment of wet AMD patients were “reduced treatment burden” (75.2%) and “long-acting sustained delivery” (66.2%), “new treatment mechanisms of action, or MOAs” (36.0%), “improved efficacy” (29.8%)and “improved safety” (11.3%).11
WET AMD TREATMENT
TENAYA and LUCERNE were two identical, randomized, multicenter, double-masked, comparator-controlled, 2-year, Phase 3 trials evaluating the efficacy, durability and safety of Vabysmo every 8-16 weeks (Q8-16W) vs 2 mg aflibercept Q8W, totaling 1,329 treatment-naïve wet AMD patients.2,12 Patients with vision 20/32 – 20/320 with subfoveal choroidal neovascularization, or juxtafoveal and extrafoveal choroidal neovascularization (CNV) with a subfoveal component were included in the study.6
Patients were randomized into two treatment arms. The first arm received four monthly doses of Vabysmo 6.0 mg followed by fixed Q8W, Q12W or Q16W intervals based on disease activity as evaluated by OCT and visual acuity at weeks 20 and 24. The second arm received aflibercept for 3 monthly doses followed by treatments Q8W.
The study met its primary endpoint of non-inferiority of Vabysmo Q8-Q16W to aflibercept Q8W in mean change of BCVA from baseline at 1 year, which was +6.2 letters vs +5.9 letters, respectively.2,13
Across both studies, 78% of patients in the Vabysmo 6.0 mg arm reached Q12W+ dosing (33% Q12W and 45% Q16W) at Year 1.2,13 In year 2, patients in the Vabysmo arm were treated according to a personalized treatment interval (PTI), extending their treatment interval in 4-week increments to a maximum of 16 weeks or reducing by 4 or 8 weeks depending on the disease activity assessments, which occurred at the active study drug treatment visits.6
In the clinical trials, subretinal fluid was tolerated in Year 2 in the Vabysmo arm, provided it did not meet a certain threshold of pre-specified disease activity. Anatomical outcomes were comparable between both arms, with reduction of central subfield thickness (CST) starting at week 4 and maintained through week 48.6 The same is true of mean change in CNV lesion size and total area of leakage compared to baseline.6
Conjunctival hemorrhage was the most common ocular adverse event during these trials.2 There were no cases of retinal vasculitis, and rates of intraocular inflammation were low (2%) for Vabsymo though numerically higher (13 to 8, respectively, pooled).6 Serious adverse events were low comparable across all arms, with one reported culture-negative endophthalmitis case in the aflibercept arm.
DME TREATMENT
YOSEMITE and RHINE were two identical, randomized, multicenter, double-masked, comparator-controlled, 2-year, Phase 3 trials evaluating the efficacy, durability, and safety of Vabysmo Q4-16W vs 2 mg aflibercept Q8W, totaling 1,891 patients with center-involving DME.14 All patients included had BCVA 20/40 – 20/320 with a CST ≥325 µm. Previously treated patients were allowed and capped at 25% (n=409). Patients were randomized 1:1:1 to three treatments arms: Vabysmo 6.0 mg Q8W, Vabysmo per PTI up to Q16W or aflibercept 2.0 mg Q8W with all arms having pre-specified loading doses.14 The PTI arm was a protocol driven, treat-and-extend model, intended to mimic real-world treatment.
In both trials, Vabysmo achieved its primary endpoint of non-inferiority in mean change from baseline in BCVA averaged over weeks 48, 52 and 56, which was +11.2, +11.2 and +10.5 letters in the three treatment arms, respectively. More than 70% of Vabysmo patients in the PTI arm reached Q12W or longer interval dosing, with more than 50% of patients reaching Q16W treatments through year 1.14
Though all arms showed significant reductions in CST, the Vabysmo-treated patients performed somewhat better than the aflibercept arm in this regard. Furthermore, a greater proportion of patients receiving Vabysmo compared to aflibercept had absence of intraretinal fluid at week 56.
All treatment arms demonstrated comparable two-step improvement from baseline in their respective ETDRS-DRSS. However, these were prespecified secondary endpoints and were not powered adequately to draw definitive conclusions.
Shortly following The Lancet publication, the year 2 results demonstrated maintenance of the vision gains achieved at year 1 throughout year 2 across the three treatment arms. Furthermore, the improved durability with nearly 80% of patients who achieved Q16W dosing at year 1 did not diminish in year 2 in the PTI arms of both studies, as well as the anatomic benefit with VABYSMO as compared to aflibercept through year 2.
Vabysmo was comparable to aflibercept in terms of safety. There were no incidents of retinal vasculitis or occlusive retinal vasculitis. The most common ocular adverse event across all four trials was subconjunctival hemorrhage.
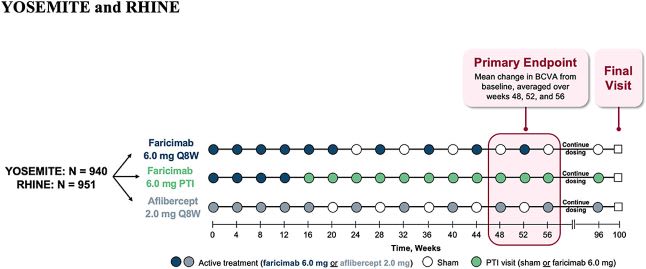
Yosemite/Rhine trial design where patients were randomized 1:1:1 to the three treatment arms.15 Image courtesy: Genentech

Vabysmo (Q8W and PTI) shows rapid and sustained visual gains through one year and met its primary endpoint vs aflibercept Q8W in the Yosemite/Rhine trials.16 Image courtesy: Genentech
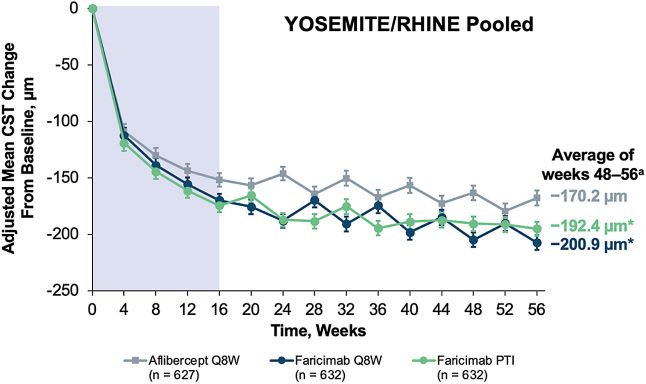
Reductions in CST were observed across all treatment arms.16 Image courtesy: Genentech
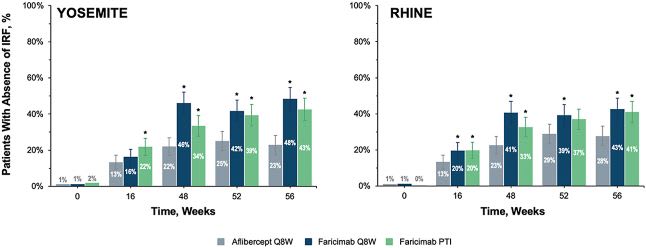
Absence of IRF was studied across all treatment arms.16 Image courtesy: Genentech
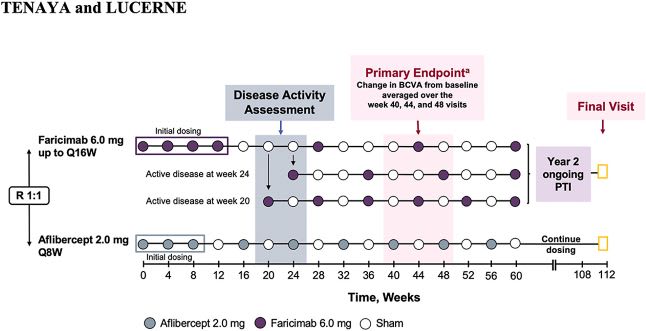
Tenaya/Lucerne trial design where patients were randomized 1:1 to the two treatment arms.17 Image courtesy: Genentech
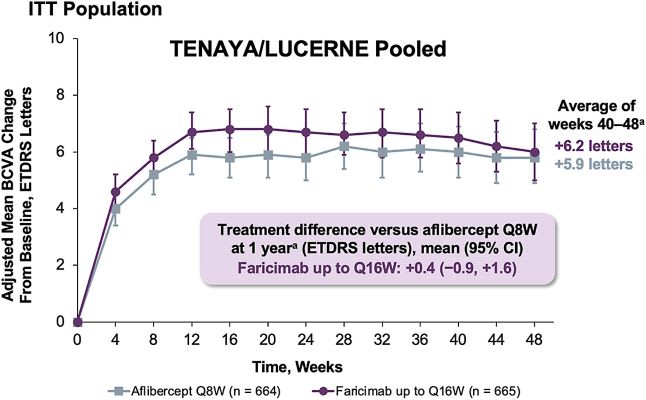
Vabysmo Q8-16W shows rapid and sustained visual gains through one year and met its primary endpoint vs aflibercept Q8W in the Tenaya/Lucerne trials.18 Image courtesy: Genentech
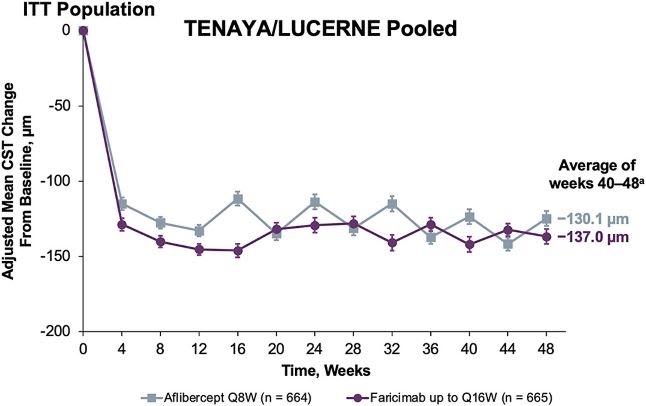
Reductions in CST were observed across all treatment arms.18 Image courtesy: Genentech
PERSONAL EXPERIENCE WITH VABYSMO
Many of us are quite cautious and conservative when it comes to treating our patients, especially with new medications with a novel mechanism of action. Many of our patients come to our offices frequently for their injections, and we view many of them as extensions of ourselves. The vast majority of my Vabysmo-treatment patients suffer from wet AMD and are being injected less than Q6W, though I have started Vabysmo on one treatment-naïve patient with nAMD.
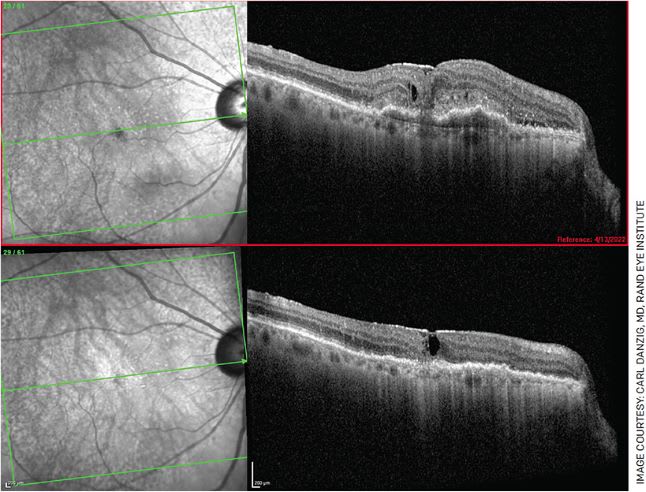
FIGURE 1. A 76-year-old female who complained of new-onset blurry vision for 3 weeks, presented with 20/200 vision in her right eye with CME, SRF, PED and subretinal hyperreflective material (top). She received Vabysmo in her right eye and, 1 month later, had remarkable anatomical improvement (bottom). However, her vision only improved to 20/100. She has been re-dosed, and we are waiting for her next follow-up visit with hope of further improvement.
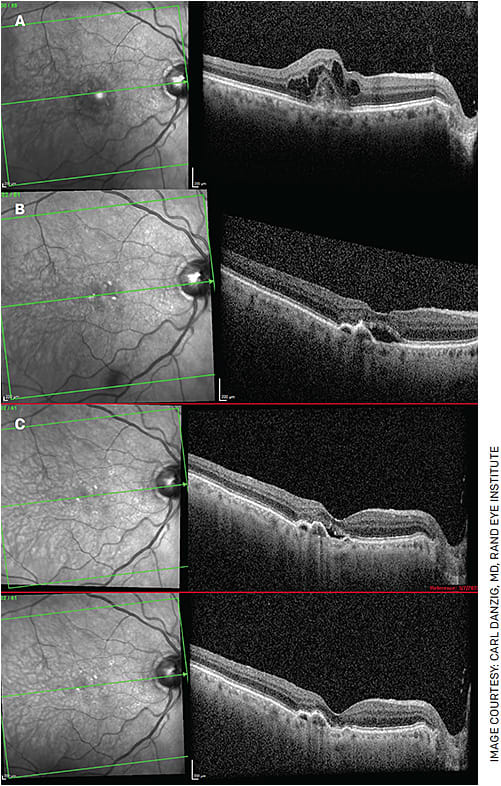
FIGURE 2. A 94-year-old gentleman has been receiving injections monthly since 2013. Figure 2A shows choroidal neovascularization with CME. This patient is highly compliant, averaging 11-12 injections per year, and was able to maintain 20/30 vision for many years. Even with a trial of Q2W injections, his subretinal fluid never resolved. Despite his frequent injections over the years, his vision declined somewhat to 20/60. Despite monthly injections, the patient had persistent subretinal fluid in February 2018 (Figure 2B). In March 2022, he received his first Vabysmo injection and, for the first time in almost 9 years, has been without subretinal fluid (Figure 2C following his first Vabysmo injection). Unfortunately, his vision remains 20/60, but he is hopeful he can extend his injection interval in the near future.
I am currently starting to treat more of my DME patients with Vabysmo as well, and the delay has been due to commercial insurance approval for many of these patients. Our diabetic patients have an exorbitant amount of doctor visits, and there is clearly an enthusiasm amongst my patient population to see me less!
I have found that Vabysmo has performed beautifully in the majority of my patients. When a patient is able to see the improvement in their OCT scan, they are excited, and my staff feels hopeful that we are able to improve the patient’s quality of life by receving fewer intravitreal injections over the course of a year.
Nevertheless, a select few patients who were dry with frequent injections showed increased disease activity 1 month after their first Vabysmo injection, and they have been switched back to their previous medications. These patients all had a rapid return to their baseline. When this occurs, I take the time to have a separate converstaion with these patients. I tell them, “I can have 10 identical plants, in the same soil, with the same nutrients and water, yet they do not all grow the same. That is biology, and the same premise applies here too, with your eye.”
Additionally, I have had zero serious adverse events in my clinic patients treated with Vabysmo.
CONCLUSION
We have fantastic medications on the market for DME and wet AMD patients, and most patients are doing well on these medications, provided they return for their regularly scheduled appointments. However, there exists an unmet need for our patients receiving the current intravitreal medications.
Vabysmo has the potential to extend the interval between their injections with increased durability and efficacy, without compromising safety. OM
REFERENCES
- Regula JT, Lundh von Leithner P, Foxton R, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases [published correction appears in EMBO Mol Med. 2019 May;11(5):]. EMBO Mol Med. 2016;8(11):1265-1288. Published 2016 Nov 2.
- VABYSMO [prescribing information]. Genentech USA, Inc.
- Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov. 2017;16(9):635-661.
- Joussen AM, Ricci F, Paris LP, Korn C, Quezada-Ruiz C, Zarbin M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye (Lond). 2021;35(5):1305-1316.
- Heier JS, Singh RP, Wykoff CC, et al. THE ANGIOPOIETIN/TIE PATHWAY IN RETINAL VASCULAR DISEASES: A Review. Retina. 2021;41(1):1-19.
- Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729-740.
- Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual Acuity Outcomes and Anti-Vascular Endothelial Growth Factor Therapy Intensity in Neovascular Age-Related Macular Degeneration Patients: A Real-World Analysis of 49 485 Eyes. Ophthalmol Retina. 2020;4(1):19-30.
- Khanani AM, Skelly A, Bezlyak V, Griner R, Torres LR, Sagkriotis A. SIERRA-AMD: A Retrospective, Real-World Evidence Study of Patients with Neovascular Age-Related Macular Degeneration in the United States. Ophthalmol Retina. 2020;4(2):122-133.
- Kiss S, Campbell J, Almony A, et al. Management and Outcomes for Neovascular Age-Related Macular Degeneration: Analysis of United States Electronic Health Records. Ophthalmology. 2020;127(9):1179-1188.
- Ciulla TA, Pollack JS, Williams DF. Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: a real-world analysis of 28 658 patient eyes. Br J Ophthalmol. 2021;105(2):216-221.
- Hahn P et al, eds. ASRS 2020 Preferences and Trends Membership Survey. American Society of Retina Specialists. https://www.asrs.org/content/documents/_2020-pat-survey-results-for-website-c.pdf . Accessed June 21, 2022.
- Khanani AM, et al. Oral presentation at Association for Research in Vision and Ophthalmology 2021.
- Singh RP, et al. Oral presentation at American Academy of Ophthalmology 2021.
- Wykoff CC, Abreu F, Adamis AP, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399(10326):741-755.
- YOSEMITE and RHINE Phase 3 Randomized Clinical Trials of Faricimab for Diabetic Macular Edema: Study Design and Rationale" by Eter N et al. is licensed under CC BY 4.0.
- Heier JS, et al. Faricimab in Diabetic Macular Edema: 1-Year Efficacy, Safety, and Durability in the Phase 3 YOSEMITE and RHINE Trials. Presented at the Annual Meeting of the American Academy of Ophthalmology. New Orleans, LA. November 12-15, 2021.
- TENAYA and LUCERNE: Rationale and Design for the Phase 3 Clinical Trials of Faricimab for Neovascular Age-Related Macular Degeneration" by Khanani AM et al. is licensed under CC BY 4.0.
- Singh RP, et al. Faricimab in Neovascular Age-Related Macular Degeneration: Primary Results From the Phase 3 TENAYA and LUCERNE Trials. Presented at the Annual Meeting of the American Academy of Ophthalmology. New Orleans, LA. November 12-15, 2021.









