Compliance is one of the biggest barriers to controlling glaucoma. In fact, a 2015 study of more than 1,200 newly diagnosed glaucoma patients who were started on topical therapy found a measly 20% had persistently good treatment adherence at 1 year.1
Data from multiple studies shows the positive impact of reducing the drop burden on progression of disease — and quality of life — post selective laser trabeculoplasty (SLT) and minimally invasive glaucoma surgery (MIGS) procedures. Benefits of these procedures include less risk for progressing to incisional glaucoma surgery to lower IOP, fewer drops necessary to achieve target IOP, less visual field loss and less likelihood of requiring cataract surgery.2,3
The recently available sustained-release intracameral bimatoprost (Durysta, Allergan) not only enables us to circumvent the issue of patient compliance with demanding and expensive drug regimens, it also enables us to avoid — or at least delay — those surgical therapies. Here are my tips for incorporating the drug into everyday clinical practice as well as presenting the option to patients.
DRAWBACKS OF THE EYEDROP REGIMEN
In addition to the usual reasons for failing to comply with the drug regimen — forgetfulness, the unsteady hands of older patients as they try to administer the drops, affordability — researchers have demonstrated that ocular surface disease (OSD) is another challenge. Up to 60% of our glaucoma patients have concomitant dry eye, which is also related to the number of topical glaucoma medications.4-6 Due to the symptoms of OSD, such as tearing, burning, pain and fluctuating vision, patients often blame their glaucoma drops on their symptoms and reduce or even stop taking their glaucoma drops. Baudouin demonstrated a 30% reduction in compliance when glaucoma patients also had a dry eye.7
Poor compliance can lead to fluctuating IOP. As studies such as AGIS in 2000 show, advanced open-angle glaucoma (OAG) patients who fluctuated more than 4 mm Hg throughout the course of the study had a higher risk of progression than those who had less fluctuation. Another study from 2019 demonstrated revealed short-term IOP fluctuation as risk factors for glaucoma progression on visual fields (VF).8
HOW DURYSTA WORKS
Since June 2020, we have had access to Durysta, which has allowed us to decrease the drop burden for a variety of patients. The indication is broad: an option for patients with ocular hypertension and OAG, regardless of disease severity or type of OAG. The 1-mm preservative-free implant infused with 10 µg of bimatoprost actively releases medication all day for 4 months. Durysta comes preloaded in an ergonomic injector system, and the FDA has approved it for a single administration.
Insertion is performed by entering the anterior chamber (AC) via the loader’s 28-g needle through the clear cornea, aiming over the iris. Once two bevel lengths into the AC, the surgeon presses an actuator button to release the implant then comes straight back out. Gravity causes the implant to sink to the inferior angle, where it slowly biodegrades into two inert substances: glycolic and lactic acid. Gonioscopy is crucial before considering Durysta. Ideally, we want to see the ciliary body band and make sure the iris doesn’t slope into the angle.
IMPLANTATION PEARLS
Durysta implantation can be performed in the office at the slit lamp or in a minor procedure room; it can also be done at the ASC or HOPD. A facility fee is associated with the procedure, which makes it palatable for ASC and HOPDs.
Regardless of where you perform the procedure, it is important to follow these three criteria:
- Use good magnification, whether loops, a slit lamp or microscope
- Provide good head stabilization (ie, have a technician hold the head still if performed at the slit lamp)
- Follow good aseptic sterile technique using betadine prep, sterile gloves and the option of peri-procedure topical antibiotics.
As an investigator in the Phase 2 and 3 trials, I implanted the drug in a minor procedure room using an OR microscope. Since commercialization, however, I have transitioned all my cases to the slit lamp. I have found the slit lamp technique flows well with my clinic schedule and provides a low-stress experience for the patient. In fact, many patients have commented the procedure was more comfortable than a “pressure check.”
I do not use a speculum or second instrument since I enter the eye between 7-8 o’clock for the right eye and 4-5 o’clock for the left eye. This way, I do not need the patient’s eyes wide open; I have them keep their eye open naturally and stare at a part of the slit lamp to provide eye stability and counter traction without using a second instrument. I anesthetize both eyes, even if only performing the procedure in one, because it helps prevent the patient from squeezing the non-procedure eye. I use the cheek bones to rest my wrist and last three fingers, which helps to stabilize my hand (Figure).
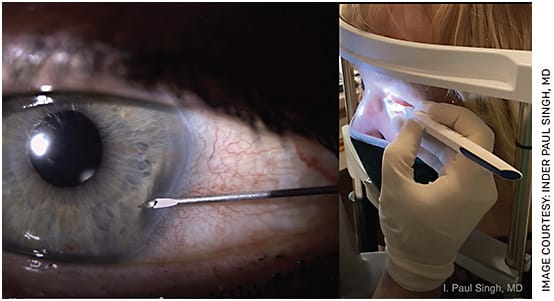
FIGURE. For insertion, I stabilize my hand by resting my wrist and last three fingers on the patient’s cheekbone.
By avoiding a speculum or second instrument, patients tend not to view the insertion as a surgery or even minor procedure; rather, they regard it as part of the routine eye exam.
SCHEDULING AND WORKFLOW
Many clinicians implant Durysta in one eye then have the patient come back for the other eye 7 to 10 days later. If a patient needs the implant in both eyes, I insert Durysta in both eyes at the same visit. I have found the patient perception of the procedure to be even more positive by having both eyes implanted the same day, which it makes it, again, more like a pressure check than a procedure, in their minds.
Important to note: Although you will receive full reimbursement for the cost of both implants, you will get 50% of the physician reimbursement for the second eye if you implant in both eyes on the same day.
Prior to me seeing the patient for the Durysta visit, technicians prep patients with 5% betadine and topical anesthetic and antibiotic drops. So, when I enter the room, the patients are already at the slit lamp, my gloves are on the table and there is very little disruption to the flow of the clinic.
Patients go home with instructions to call us in case of pain, increased redness, decreased vision or any other new symptoms. (We have not yet seen an infection, and no cases of infection were identified in the trials.)
FOLLOW UP
Patients return to the clinic 4-6 weeks later for a follow-up IOP check and gonio to check positioning of the implants. Assuming the IOP is at target, I have them return based on disease severity. I bring back mild OAG patients in 6 months and moderate to severe patients in 3-4 months. In the clinical trials, the IOPs did not return to baseline rapidly, so you should be able to identify patients as their IOPs are rising and then decide if they should restart drops or consider another treatment.
I often keep patients off drops even if the IOP is a couple mm Hg higher at the follow-up visit, especially if they are still in the target range. I would rather have a patient 1-2 mm Hg higher but without compliance issues than a patient non-compliant with drops, which could lead to fluctuating IOPs.
It is important to recognize the duration of IOP reduction following a single Durysta implantation has lasted up to 2 years in up to 25% of patients and 1 year in 36% of patients.9 It seems a potential remodeling of the target tissues occurs, including the ciliary body and trabecular meshwork.
Studies have demonstrated an increased expression of MMP (metalloproteinase) as well as a decreased expression of TIMS (tissues inhibitor of metalloproteinase) with both topical and intracameral bimatoprost, magnitude significantly higher with intracameral administration.10 This overexpression of MMP and significant inhibition of TIMS could be why we are seeing the significant duration of effect. At my practice, we find 70% of patients at 9 months and more than 45% at 1 year do need rescue therapy.
PATIENT SELECTION
So, how do we pick the right patient? I have learned the key is not to wait for the “ideal” candidate. Any patient with compliance issues could be suitable, regardless of the IOP or stability of optic nerve head and visual fields. Pay attention to clues of poor compliance, whether due to cost, side effects, forgetfulness, physical limitations or OSD. These patients are in our offices every day and routinely telling us they are not compliant, but we are often too busy to notice.
Involving the technicians can help. For instance, a patient may ask for “samples” every time they come in, indicating cost is an issue, complain of fluctuating vision, indicating OSD, or not even be able to recall the color of the top of the drop bottle, indicating forgetfulness.
Although the patient on topical PGA monotherapy is a great candidate, we have found patients on multiple drops can benefit from Durysta. Not only does it help to get them off the topical PGA, but patients on multiple drops often have poor compliance and would benefit.
I have also found post-SLT patients tend to be open to the idea of a procedure and are motivated to avoid topical drops. If their IOP has risen post SLT or is not at target after treatment, Durysta can be a good option. Similarly, when the patient’s IOP is not at target — whether after a combination cataract/MIGS or stand-alone MIGS — Durysta can help achieve that drop-free outcome for some time. We have noticed patients who had refused a MIGS procedure in the past then received Durysta ended up opting for MIGS once the drug wore off. Durysta seemed to serve as a bridge help them feel comfortable with a surgical procedure. Patients who may need a subconjunctival surgery, such as a trabeculectomy or XEN (Allergan), and have significant ocular surface inflammation can benefit from Durysta, allowing us to remove the topical prostaglandin analogue (PGA) in hopes of decreasing surface inflammation and thus reduce risk of post op bleb failure.
PRESENTING IT TO THE PATIENT
Once you identify a patient with a compliance issue, use that as the rationale to discuss Durysta. You are offering a solution to their problem.
When describing the procedure, I do not use the words “needle” or “injection.” I say something like, “Mrs. Smith, that drop you are taking at night seems to be causing some redness [or insert any other compliance issue for that patient]. I can help you. There is a product available that has the equivalent of one drop of that medication in a small, dissolvable pellet. Right here, I can press a button and gently place the pellet in your eye. The pellet releases the medication 24 hours a day for 4 months. The effect of the medication can last even longer, up to 2 years in some patients. We will follow you like we do now, and when the pressure comes back up we can restart medications or consider alternative options. I think this would help you with the issues you are having and would give you a break from that drop.”
DURATION AND SAFETY
Some clinicians are concerned with the single-administration approval, feeling it is a barrier for adoption. But it’s all about the risk-benefit ratio. I tell patients there is no cure for glaucoma and no treatment that lasts forever. At any given time, I recommend various therapies to help protect them from losing vision and maintain a high quality of life.
Because the safety and patient experience are so positive with Durysta, I find the expectation of duration of effect is not as high as for a surgical option. When we offer SLT, we tell patients it works 80% of the time, the effect is like that of a topical drop and the effect can last anywhere from a few months to a few years. It is a similar discussion with Durysta, although my practice has found well over 90% of patients have significant efficacy.
Once they understand the risks and benefits, patients don’t seem to be turned off with Durysta being a temporary solution. Patients tell me the insertion is easier than getting their pressures checked. To this day, I have not had a patient regret having Durysta implanted even after the effect wore off. They routinely thank me for the time they had off the drop.
REDUCING IOP, INCREASING QUALITY OF LIFE
With sustained-release drug delivery and our other interventions, we are now better equipped than ever to reduce IOP earlier in the disease while maintaining a high quality of life. The break from a drop regimen that a sustained-release drug delivers benefits to the practice as well as the patient in the form of fewer call backs, less time faxing to the pharmacy and wrangling with insurance companies, and less time verifying medications at the patient’s office visits.
It’s about time we get ourselves and our patients off the glaucoma treatment hamster wheel. OM
REFERENCES
- Newman-Casey PA, Blachley T, Lee PP, Heisler M, Farris KB, Stein JD. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015;122:2010-2021.
- Gazzard G, Konstantakopoulou E, Garway-Heath D, et al; LiGHT Trial Study Group. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393:1505-1516.
- Ahmed II, DeFranceso T, Rhee D, et al, for the HORIZON Investigators. Long term outcomes from the HORIZON randomized trial for a Schlemm’s canal microstent in combination cataract and glaucoma surgery. Ophthalmol. Epub ahead of print, Feb. 23, 2022. https://doi.org/10.1016/j.ophtha.2022.02.021 . Accessed June 24, 2022.
- Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618-621.
- Erb C, Gast U, Schremmer D. German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol. 2008;246:1593-1601.
- Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disese in glaucoma patients. J Glaucoma. 2008;17:350-355.
- Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmologica. 2008;86:716–726. 2. Stringham J et al. Eye & Contact Lens. 2018;44:50-54
- Matlach J, Bender S, König J, Binder H, et al. Investigation of intraocular pressure fluctuation as a risk factor of glaucoma progression. Clin Ophthalmol. 2018;13:9-16. Published 2018 Dec 18.
- Craven ER, Walters T, WC, et al, for the Bimatoprost SR Study Gorup. 24-month phaseI/II clinical trial of bimatoprost sustained-release implant (Bimatoprost SR) in glaucoma patients. Drug. 2020;80:167-179.
- Adapted from Yamada et al. BMC Ophthalmol. 2016;16:26 and used under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/ ) MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase









