As the technology surrounding premium IOLs has advanced at seemingly warp-speed, the options available to treat our patients have expanded significantly. Deciding which IOL is best for which patient depends on several factors — patient career, hobbies, anatomy and past ocular history all play important roles.1-7 A thorough understanding of the types of IOL technologies available, as well as their compatibilities and limitations, allows us to best match our patients’ expectations while still optimizing their visual result.
This article will focus on several case-based approaches on how to best meet our patients’ expectations utilizing the technologies available.
CASE 1
The situation
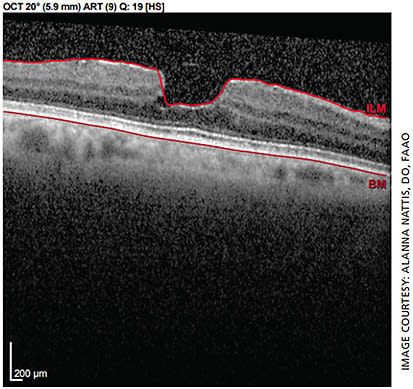
The patient is a 70-year-old healthy female with unremarkable preoperative exam in the right eye, mild epiretinal membrane (ERM) noted in the left (Figure 1). She states she desires spectacle independence post-cataract surgery.
Proposed management
It is important to remind patients that most presbyopia-correcting IOLs (PC-IOLs) provide significantly reduced spectacle dependence vs complete spectacle independence.1,2,4,5,8-16 This is important to clarify preoperatively and ensure patient understanding. That being said, as there are no noted macular pathologies, ocular surface disease or topographic abnormalities present, this patient’s right eye is an excellent candidate for a multifocal IOL that provides a full range of vision at distance, intermediate and near (eg, PanOptix, Alcon; Tecnis Synergy, Johnson & Johnson Vision).3-5,10,11,13,16-19
However, due to presence of ERM in the left eye, the surgeon should be wary of choosing an IOL with a diffractive technology, as this could further degrade vision, decrease contrast sensitivity and lead to a suboptimal result.1,6,15,20,21 The conservative way of managing this: Use a monofocal IOL in the left eye — though the patient may not feel she is as “free” from spectacles as she would like.1,6,21,22 This should be communicated to the patient prior to surgery so that her expectations are on par with the surgical plan.
Another option is a non-diffractive extended depth of focus (EDOF) IOL in the left eye, such as Vivity (Alcon). This lens is designed to perform similarly to a monofocal in terms of contrast sensitivity and visual aberrations with expanded visual range.6,9,12,23
It is important to educate the patient on the time required for neuroadaptation to both of these technologies, as well as that she may notice a difference between the two eyes in terms of contrast and sharpness of vision due to IOL design as well as ocular pathology.1,2,5,6,8,12,15,24,25
Alternatively, monovision could be offered to the patient; perform a contact lens trial first to ensure the patient is agreeable to this technique.1,22
Finally, an accommodating IOL such as the Crystalens (Bausch + Lomb) may be a good option to implant bilaterally or unilaterally with minimal to no potential visual aberrations. However, keep in mind it would be important to educate the patient that accommodation levels with this IOL may be much lower in comparison to other multifocal IOLs, and therefore spectacle dependence may be greater for near activities.1,8,26
CASE 2
The situation
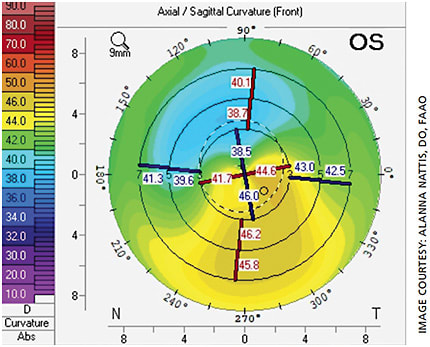
A 67-year-old male comes in for a cataract consult. He had LASIK to correct myopia 15 years prior but does not remember the name of his refractive surgeon; preoperative records are not available. He wishes to have spectacle independence post-cataract surgery. His topography is within normal limits in the right eye; the left eye shows signs of mild ectasia (Figure 2).
Proposed management
This case is challenging for multiple reasons: The patient is post-LASIK without preoperative records, one eye has ectasia and it is unclear if the ectasia is stable in that eye. With any post-LASIK patient, a scrutinizing examination of topography, tomography and higher-order aberrations (HOA) is important — especially if the patient wishes to consider a diffractive premium IOL technology, as these eyes may also have aberrations on their own.1,27,28 Though we do not have preoperative keratometry values or refraction, we are fortunate in that post-refractive surgery calculators, such as the ones provided by ASCRS (iolcalc.ascrs.org ), are available to help best calculate the appropriate IOL power.27,28
Additionally, intraoperative aberrometry can be used to confirm the IOL at time of surgery (eg, ORA, Alcon). In this case, counsel the patient on increased risk of glare, haloes and starburst. Decreased contrast sensitivity with diffractive multifocal IOLs is another potential problem to warn about due to past history of laser vision correction.2,6,27,28 Missed refractive result is another possibility due to history of LASIK; counsel the patient regarding this, in addition to providing options postoperatively in case the refractive target is missed (eg, glasses, IOL exchange or further laser vision correction enhancement for the right eye).2,6,27,28
In general, it is recommended for a total HOA of less than 0.3 µm to plan for optimal results with a multifocal IOL.27,28 Devices such as the Pentacam (Oculus), OPD-3 (Marco) and iTrace (Tracey Technologies) can be helpful for measuring HOA. If the patient is willing to accept the risk associated with multifocal IOL implantation, has been informed that an IOL exchange can be performed (if necessary) and has been educated regarding the technology used for multifocal IOLs (eg, PanOptix, Synergy), then it may be reasonable to proceed with one of these IOLs in the right eye.6,8,11,13,17-19,27,28
Alternatively, an EDOF IOL (Vivity or Symfony) may be used with slightly lower risk of visual aberrations. However, near vision may be compromised.4,6,8,10,12,15,23,25,27,28
Due to ectasia in the left eye, a multifocal/trifocal IOL is not recommended; I would be extremely hesitant about implanting an EDOF IOL in this eye as well.27-29 It is imperative to determine if the ectasia is stable in the left eye. Though it is unusual to have ectasia progression after age 40-45, it can occur. In the case of progressive ectasia, corneal crosslinking should be performed to halt progression.30,31
If it is determined that this patient’s ectasia is progressive, crosslinking should be performed to ensure a stable refractive target for cataract surgery. In the case of stable ectasia, crosslinking is therefore not indicated prior to any further surgical intervention. Once the cornea has been determined to be stable (which can take several months, if crosslinking is required), it is time to begin planning for cataract surgery. IOL options for the left eye include toric monofocal IOLs. Envista IOLs (Bausch + Lomb) come in higher toric powers, which can be very helpful in higher levels of corneal cylinder, and Alcon and Johnson & Johnson Vision both offer toric IOLs that have also been documented to give good results in these patients.29,31
In the case of stable but severe/irregular ectasia, it may be best to implant a monofocal (non-toric IOL) and have the patient fitted for a contact lens postoperatively; topography-guided PRK may be an option in some cases as well.31
The patient should be informed that if he decides to pursue cataract surgery with a presbyopia-correcting IOL in one eye and a monofocal IOL in the left eye (spherical or toric), he may experience some issues with neuroadaptation and adjusting to depth perception.1,2,21,22,24 In addition, in some ectasia cases, use of a toric IOL may be associated with increased risk of postoperative IOL rotation due to deeper anterior chambers (and associated steeper keratometry values).29
CASE 3
The situation
A 55-year-old female with a reportedly normal preoperative exam had successful cataract surgery with bilateral trifocal IOL implantation by an outside surgeon. However, she comes in for a second opinion 2 weeks postop because she is experiencing “very bothersome” glare and haloes that started immediately after surgery. She says they are fairly equal between the two eyes.
Proposed management
Whenever a patient is dissatisfied after cataract surgery, understanding the nature of the complaint is critical.6,8,9,24,32 This patient had reportedly uneventful surgery with trifocal IOL implantation. Elaboration of her symptoms is important here — are her glare and haloes mainly nocturnal in nature or do they occur during the day as well? A thorough ocular exam, including uncorrected and best-corrected visual acuity, as well as an anterior and posterior segment exam should be performed. It is important to remind the patient that often glare and haloes will diminish over time post-surgery. As the eye heals and neuroadaptation occurs, the patient may not notice these visual aberrations much or at all.6,9,24
In addition, during this early postoperative healing period, use of topical brimonidine as needed for glare/haloes has been documented to help reduce nocturnal visual aberrations.32,33 However, it is also important to search for other causes of visual disturbances.
Occasionally, residual refractive error can lead to visual aberrations. If this is the case, explore how to best treat this — whether by glasses, contact lenses or laser vision correction. Inspection of the ocular surface is also incredibly important as even mild dry eye can cause fluctuations in vision; the presence of a diffractive IOL can accentuate these disturbances — both during the day and at night (Figure 3).6,8,9,32
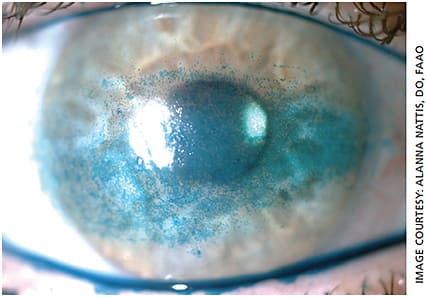
Intensive treatment of ocular surface disease as well as meibomian gland dysfunction/blepharitis is key for all cataract surgery patients to best optimize vision. Inspection of the posterior cornea is important here too — undiagnosed corneal dystrophy, such as Fuchs’ or posterior polymorphous dystrophy, may contribute to glare/haloes on its own; this effect is amplified with implantation of a diffractive multifocal IOL. If present, the patient should be properly educated regarding options for management, which may include IOL exchange and/or corneal transplant surgery in severe cases (eg, Descemet’s membrane endothelial keratoplasty). This is an important conversation to have as medical treatment alone will not eliminate the visual aberrations caused by the corneal dystrophy.
Centration of the IOL is also important. Even slight decentration or malposition can lead to increased higher-order aberrations and visual dissatisfaction — this is especially true for toric multifocal IOLs.6,9 When possible, IOL decentration should be managed early and surgically in order to best optimize vision. If the capsular bag is compromised and the IOL is unstable, the patient should be informed of the need for IOL exchange for a monofocal, as a compromised capsular bag will ultimately lead to further IOL decentration and worsening visual aberrations and decline.
Presence of early posterior capsule opacification is one of the most common causes of visual disturbances post-multifocal IOL implantation.6,9 If this is the case, educate the patient on the option of YAG capsulotomy to help improve symptoms. However, it is advised to wait at least 2 to 3 months postoperatively to ensure the patient is otherwise satisfied with the extended range of vision from the IOL, as IOL exchange post-YAG is significantly more difficult to perform.
Finally, it is rare for retinal pathology to cause the bilateral glare and haloes this patient is experiencing, but inspection of the posterior segment should always be performed to ensure there are no other anatomical causes or risks of visual decline postoperatively.
CASE 4
The situation
A 70-year-old male presents for cataract evaluation. He has a 60-prism-diopter exotropia in the right eye with history of dense amblyopia in that eye since childhood (BCVA is 20/100). His ocular exam is otherwise normal with good potential visual acuity in the left eye. He wishes to have spectacle independence post-cataract surgery.
Proposed management
This patient requires extensive counseling to ensure he understands that cataract surgery will not greatly improve the vision nor strabismus in his right eye. In addition, discussion regarding strabismus surgery and possible diplopia is encouraged should the patient need to pursue strabismic correction post cataract surgery. His left eye, on the other hand, is a good candidate for a PC-IOL. Discussion of the different types of IOLs available (trifocal, EDOF, accommodating, light-adjustable) is appropriate. As this patient wishes to be less spectacle dependent, discussion may revolve more around available trifocal vs. EDOF IOL options.
It is important to highlight benefits and potential disadvantages of each technology; trifocal IOLs provide the most spectacle independence, while a non-diffractive extended vision IOL such as Vivity may provide a good range of vision (excellent distance and intermediate with adequate near) with minimal potential adverse effects, such as glare and haloes.2,8,10,12,13,17-19,23
Discussion of risk for IOL exchange due to visual disturbances is necessary — surgeons should try to avoid excess or avoidable surgeries on monocular patients such as this. Therefore, education and determination of the patients’ postoperative desires must be clear.
Another reasonable option would be to place a monofocal IOL in the right eye and a Light Adjustable Lens (LAL, RxSight) in the left eye, which can provide sharp distance vision, with the option for adjustment should residual refractive error remain postoperatively.30,31 This option, however, would not provide spectacle independence, as glasses would be required for near activities.
CASE 5
The situation
A 60-year-old female police officer with an otherwise normal eye exam developed bilateral posterior subcapsular cataracts. The impact on her ability to work is significant. As a result, she spends time doing both deskwork and fieldwork and desires spectacle independence so that she has a good range of vision during both day and night with minimal risk of glare/haloes.
Proposed management
This patient is a good candidate for PC-IOL implantation — but within reason. She states outright she does not want to risk glare and haloes, which puts diffractive IOL technology lower on the list of options for her. That leaves a choice between a non-diffractive EDOF IOL (eg, Vivity), which offers a visual disturbance profile similar to that of a monofocal (though she will likely need reading glasses for near work), or monovision.3,4,5,7,18,19,21,22,32 Should she choose monovision, a contact lens trial should be performed prior to surgery to ensure this is a good option for her.
In addition, the patient should be informed of the possible need for glasses while driving at night, as monovision may impair depth perception, especially in low-lighting conditions.22 In the case of monovision cataract surgery, use of the LAL may be helpful to ensure a precise refractive outcome.34,35
The patient should be educated about the need for additional postoperative visits with the associated light delivery device to “lock-in” the IOL power and the need to avoid excessive UV light exposure for a specified period of time postoperatively.34,35
LET THE CRITERIA BE YOUR GUIDE
With our expanding options in premium IOL technology, choosing the “right match” for the right patient can sometimes be a challenge. However, breaking options down into a categorized approach — according to patient desires and lifestyle, patient anatomy, potential advantages and disadvantages of the IOL technologies available — will allow for a logical and effective strategy to optimize visual outcomes. OM
REFERENCES
- Khandelwal S, Jun J, Mak S, et al. Effectiveness of multifocal and monofocal intraocular lenses for cataract surgery and lens replacement: a systematic review and meta-analysis. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2019; 257: 863-875.
- De Silva SR, Evans JR, Kirthi V, et al. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Syst Rev. 2016; 12: CD003169
- Rosen E, Alio JL, Dick H, et al. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange. Meta-analysis of peer-reviewed publications. J Cataract Refract Surg. 2016;42:310-328.
- Pedrotti E, Carones F. Aiello F, et al. Comparative analysis of visual outcomes with four intraocular lenses: Monofocal, multifocal, and extended range of vision. J Cataract Refract Surg. 2018; 44:156-167.
- De Mederios AL, de Araujo Rolim AG, Pimenta Motta AF, et al. Comparison of visual outcomes after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of an extended depth of focus intraocular lens with a diffractive bifocal intraocular lens. Clin Ophthalmol. 2017;11:1911-1916.
- Gibbons A, Ali TK, Waren DP, Donaldson KE. Causes and correction of dissatisfaction after implantation of presbyopia-correcting intraocular lenses. Clin Ophthalmol. 2016; 10:1965-1970.
- Beltz J. The premium IOL pipeline. Ophthalmology Management. 2020; 24:34, 37,38,40.
- Rudalevicius P, Lekaviciene R, Auffarth GU, et al. Relations between patient personality and patients’ dissatisfaction after multifocal intraocular lens implantation: clinical study based on the five factor inventory personality evaluation. Eye. 2020; 34: 717-724.
- Sachdev GS, Sachdev Mahipal. Optimizing outcomes with multifocal intraocular lenses. Indian Journal of Ophthalmology. 2017; 65:1294-1300.
- Cochener B, Boutillier G, Lamard M, Auberger-Zagnoli C. A comparative evaluation of a new generation diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34:507-514.
- Kohnen T, Marchini G, Alfonso JF. Innovative trifocal (quadrifocal) presbyopia-correcting IOLs: 1-year outcomes from an international multicenter study. J Cataract Refract Surg. 2020; 46:1142-1148.
- Singh B, Sharma S, Dadia S, et al. Comparative evaluation of visual outcomes after bilateral implantation of a diffractive trifocal intraocular lens and an extended depth of focus intraocular lens. Eye Contact Lens. 2020;46:314-318.
- Kohnen T, Herzog M, Hemkeppler E, Schonbrunn S, DeLorenzo N, Petermann K, Bohm M. Visual Performance of a Quadrifocal (Trifocal) Intraocular Lens Following Removal of the Crystalline Lens. Amer J Ophthalmology; 2017; 184: 52-62.
- Vilar C, Hida WT, deMedeiros A, et al. Comparison between bilateral implantation of a trifocal intraocular lens and blended implantation of two bifocal intraocular lenses. Clin Ophthalmol 2017; 11:1393-1397.
- Monaco G, Gari M, Di Censo F, et al. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg 2017; 43:737-747.
- Kohnen T, Titke C, Bohm M. Trifocal intraocular lens implantation to treat visual demands in various distances following lens removal. Am J Ophthalmol. 2016; 161:71-77.
- TECNIS Synergy Intraocular Lens, TECNIS Synergy Toric II Intraocular Lens, TECNIS Synergy IOL with TECNIS Simplicity Del. Premarket Approval (PMA) FDA. US Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P980040S124 .
- Summary of Safety and Effectiveness Data (SSED) TECNIS SYNERGY IOLs, Models ZFR00V, DFR00V, ZFW150, ZFW225, ZFW300, ZFW375, DFW150, DFW225, DFW300 and DFW375. FDA. https://www.accessdata.fda.gov/cdrh_docs/pdf/P980040S124B.pdf
- Summary of Safety and Effectiveness Data (SSED) Multifocal Intraocular Lens (AcrySof IQ PanOptix Trifocal Intraocular Lens (Model TFNT00), Acrysof IQ PanOptix Toric Trifocal Intraocular Lens (Model TFNT30, TFNT40, TFNT50, TFNT60). FDA. https://www.accessdata.fda.gov/cdrh_docs/pdf4/P040020S087B.pdf
- Fraser-Bell S, Guzowski M, Rochtchina E, Wand J, Mitchell P. Five-year cumulative incidence and progression of epiretinal membranes. Ophthalmology. 2003; 110:34-1140.
- Cionni R, Osher R, Snyder M, Nordlund M. Visual outcome comparison of unilateral versus bilateral implantation of apodized diffractive multifocal intraocular lenses after cataract extraction: prospective 6-month study. J Cataract Refract Surg. 2009; 35:1033-1039.
- Smith C, Wilcox R, Allison S, Karanovic O, Wilkinson F. Monovision: Consequences for depth perception from fine and course disparities. ARVO Annual Meeting Abstract. April 2009.
- Summary of safety and effectiveness data (SSED) extended depth of focus intraocular lens (Acrysof IQ Vivity Extended Vision Intraocular Lens (Model DFT015), Acrysof IQ Vivity Toric Extended Vision IOLs (DFT315, DFT415, DFT515). FDA https://www.accessdata.fda.gov/cdrh_docs/pdf/P930014S126B.pdf
- Rosa A, Miranda A, Patricio MM, et al. Functional magnetic resonance imaging to assess neuroadaptation after bilateral cataract surgery. Ophthalmol. 2013;120(12): 2449-2455.e1
- Savini G, Schiano-Lomoriello D, Baducci N, Barboni P. Visual performance of a new extended depth of focus intraocular lens compared to a distance-dominant diffractive multifocal intraocular lens. J Refract Surg. 2018; 34: 228-235.
- Schena LB. IOLs for presbyopia move ahead. American Academy of Ophthalmology. 2010. https://www.aao.org/eyenet/article/iols-presbyopia-move-ahead . Accessed Sept. 2, 2021.
- Alfonso JF, Madrid-Costa D, Poo-Lopez A, Montes-Mico R. Visual quality after diffractive intraocular lens implantation in eyes with previous myopic laser in situ keratomileusis. J Cataract Refract Surg 2008;34:11:1848–1854.
- Muftuoglu O, Dao L, Mootha VV et al. Apodized diffractive intraocular lens implantation after laser in situ keratomileusis with or without subsequent excimer laser enhancement. J Cataract Refractive Surg 2010;36:11:1815-1821.
- Allard K, Zetterberg M. Implantation of toric intraocular lenses in patients with cataract and keratoconus: a case series. Int Med Case Rep J. 2018; 11:185-191.
- Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. May 2003;135:620–627.
- Nattis AS, Rosenberg ED, Donnenfeld ED. One-year visual and astigmatic outcomes of keratoconus patients following sequential crosslinking and topography-guided surface ablation: the TOPOLINK study. J Cataract Refract Surg. 2020 Apr;46:507-516.
- Bizer W. How can I reduce halo and glare from my multifocal lenses after cataract surgery? American Academy of Ophthalmology. 2013. https://www.aao.org/eye-health/ask-ophthalmologist-q/halo-cataract-surgery-multifocal . Accessed Sept. 1, 2021.
- Lee J, You Y, Choe C, Lee E. Efficacy of brimonidine tartrate 0.2% ophthalmic solution in reducing halos after laser in situ keratomileusis. J Cataract Refract Surg. 2008; 34:963-967.
- Summary of Safety and Effectiveness Data (SSED) Posterior Chamber IOL, Ultraviolet (UV) Light Source/Light Adjustable Lens (LAL)/Light Delivery Device (LDD). https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160055B.pdf . Accessed Sept. 1, 2021.
- Achieving customized vision with the light adjustable lens. https://www.rxsight.com/us/customizing-your-vision . Accessed July 9, 2021.









