During the last 25 years of Ophthalmology Management, we have covered every significant development and innovation to occur within the industry. To celebrate these advancements and events, we asked our readers, members of industry and our editorial board to nominate what they feel were the most impactful equipment, products, techniques and practice changes to occur during this period. With input from our editorial advisory board, we narrowed down the nominees to a list of 25. Below, key opinion leaders highlight why each development belongs on this list and share their perspective on its impact on the industry and patient care.
FEMTOSECOND LASER-ASSISTED CATARACT SURGERY
MINIMALLY INVASIVE GLAUCOMA SURGERY
PUPIL TRACKING AND REGISTRATION SYSTEMS
ROUTINE SURGERY FOR EPIRETINAL MEMBRANES
AFFORDABLE CARE ACT
BY DAVID GLASSER, MD
The Patient Protection and Affordable Care Act (ACA), also known as Obamacare, represents the most comprehensive change to the US health-care system since Medicare was created in 1965. It was signed into law on March 23, 2010, was implemented over the ensuing 4 years and has withstood intense and repeated political and legal challenges.
ACA’s primary goals were to expand health insurance coverage and reform care delivery to control costs and increase quality. It reduced the number of uninsured Americans by almost half and slowed the increase in health-care costs.1,2 This was achieved by creating insurance exchanges with subsidized premiums and expanding Medicaid eligibility. Far-reaching changes to private insurance were mandated, including requirements to cover essential health benefits and elimination of pre-existing condition exclusions.
Vertical integration of health care around HMOs, hospitals and Accountable Care Organizations was encouraged by ACA. Ophthalmology, with a high percentage of Medicare patients and minimal contribution to hospital revenue, was somewhat insulated from those trends at the time.
ACA ushered in an era of high-deductible health plans. Those who opted for low-premium plans were often stunned by the size of their deductibles (over $5000 for Bronze plans). This prompted some patients to forego care, while others left practices with significant accounts receivable. One policy twist continued coverage for beneficiaries who failed to pay their premiums 2 months beyond the time that carriers were required to pay providers, leaving physicians with no recourse for reimbursement.
Much of the ACA’s impact on ophthalmologists was related to commercial carriers’ responses to participation in the health insurance exchanges. Faced with increased coverage requirements, new beneficiaries needing more care and reduced premiums, they reduced their cost of care by narrowing networks, increasing pre-authorizations and limiting formularies across all lines of business. This increased administrative burdens for ophthalmologists and created barriers to specialty care.
ACA directly increased recordkeeping and reporting burdens by expanding value-based purchasing. Later legislation like the HITECH Act and MACRA created additional burdens, which are at times mistakenly attributed to ACA.
Expanded insurance coverage under ACA has been largely beneficial, while the Academy advocates for relief from the associated administrative burdens.
REFERENCES
- U.S. Department of Health & Human Services. gov. New HHS Data Show More Americans than Ever Have Health Coverage through the Affordable Care Act. https://www.hhs.gov/about/news/2021/06/05/new-hhs-data-show-more-americans-than-ever-have-health-coverage-through-affordable-care-act.html . Accessed October 11, 2021.
- Vangipuram Suresh K, Wang K, Margalit A, Jain A. Trends in Out-of-Pocket Healthcare Expenses Before and After Passage of the Patient Protection and Affordable Care Act. JAMA Netw Open. 2021;4(4):e215499. Published 2021 Apr 1. Accessed October 11, 2021.
ANTI-VEGF
BY DAVID A. EICHENBAUM, MD
Vascular pathology was revolutionized by Judah Folkman when he first published that tumor growth is dependent on the progression of tumor vasculature.1 Later work defined that such vasculature is nurtured by specific cytokines, and vascular endothelial growth factor (VEGF), initially termed vascular permeability factor (VPF), was subsequently identified in the 1980s.2
There had been suspicion since the 1950s that ischemic retina was the cause of vascular retinal pathology,3 but this was quantified to be mediated by VEGF in primate models in the 1990s.4 Although there was immediate and intense controversy about the relative potential efficacy5 and safety of ocular VEGF blockade, there was enough compelling data to try uncontrolled bevacizumab in a small series of patients, initially intravenously, in the treatment of neovascular AMD. There were immediate and striking results.6 Retina specialists almost immediately began to inject off-label bevacizumab intravitreally,7 which led to the well-developed, well-researched and well-documented anti-VEGF era in which we currently exist.
Millions of patients with common retinal diseases have had their vision saved and their lives changed forever thanks to anti-VEGF therapy. If we have a single retinal discovery in the next 25 years even half as impactful as the development of intravitreal anti-VEGF, we will have had a successful generation.
REFERENCES
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186.
- Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309-1312.
- Wise GN. Retinal neovascularization. Trans Am Ophthalmol Soc. 1956;54:729-826.
- Miller JW, Adamis AP, Shima DT, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994;145:574-584.
- Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480-1487.
- Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035-1047.
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331-335.
BIMATOPROST IMPLANT
BY E. RANDY CRAVEN, MD, FACS
In the 1970s, ocular drug delivery systems (DDS) first made their way into the therapeutic armamentarium with the contact lens-like pilocarpine polymer-membrane unit (Ocusert, Alza Corp) for the treatment of open-angle glaucoma (OAG).1,2 This paved the way for other ocular DDS such as ganciclovir (Vitrasert, pSivida) in the 1990s for cytomegalovirus retinitis and the cyclosporine implant (early 2000s) for ocular graft-versus-host disease.3-5 Then came the fluocinolone implant (Retisert, Bausch & Lomb), dexamethasone intravitreal implant (Ozurdex, Allergan) and fluocinolone acetonide intravitreal implant (Iluvien, Alimera Sciences) for retinal diseases. However, until recently there were no options for sustained-release delivery systems in glaucoma.6
The bimatoprost implant (Durysta, Allergan) has been a long time coming, with a decade of bench safety testing preceding the initiation of Phase 1 trials 10 years ago. The bimatoprost implant received FDA approval in March 2020 as a biodegradable single intracameral administration per eye and is indicated to reduce IOP in patients with OAG or ocular hypertension.7,8 The implant contains 10 µg of bimatoprost, approximately the same amount of drug as found in a single drop of Lumigan 0.03% (Allergan). Randomized controlled trials are currently ongoing to investigate the potential for implant readministration.9,10 In the Phase 3 controlled studies, the most common ocular adverse reaction (AR) reported by 27% of patients was conjunctival hyperemia.8,11 Other ARs reported in 5-10% of patients included foreign body sensation, eye pain, corneal endothelial cell loss and iritis.8,11
As the first sustained-release implant in glaucoma, the bimatoprost implant provides 24/7 targeted drug delivery to lower IOP for several months.12 With intracameral administration, the bimatoprost implant bypasses the ocular surface, thereby reducing the exposure of off-target tissues to bimatoprost.13 Patients treated in the Phase 3 trials included those on zero to three medications and those with a history of prior selective laser trabeculoplasty procedures.14-16 The bimatoprost implant can be administered outside of the OR, which may offer convenience to both physicians and patients. Sustained delivery implants can make an impact, potentially reducing the need for adherence.11,12,17
The bimatoprost implant has been on the market for over a year now, and additional sustained delivery intracameral implants and systems are in development (ENV515 travoprost implant, Envisia Therapeutics; iDose travoprost implant, Glaukos; OTX-TIC travoprost implant, Ocular Therapeutix; polycaprolactone implant with PGE2-derivative; DE-117, Santen; and latanoprost free acid SR implant, PolyActiva).18,19 Some involve new delivery vehicles and new molecules for IOP reduction. These sustained delivery systems have the capability to control IOP for an extended duration of time, and current preliminary IOP reduction data has been positive for these prospects.18,20-25 As more clinical studies on ocular DDS are completed, we will build on our understanding of how they impact patient benefits and the paradigm of glaucoma care.

REFERENCES
- Macoul KL, Pavan-Langston D. Pilocarpine ocusert system for sustained control of ocular hypertension. Arch Ophthalmol. 1975;93(8):587-590.
- Pollack IP, Quigley HA, Harbin TS. The Ocusert pilocarpine system: advantages and disadvantages. South Med J. 1976;69(10):1296-1298.
- First eye implant approved. AIDS Alert. 1996;11(5):59.
- Ganciclovir implants (Vitrasert). Treat Rev. 1996(21):10.
- Kim H, Csaky KG, Gilger BC, et al. Preclinical evaluation of a novel episcleral cyclosporine implant for ocular graft-versus-host disease. Invest Ophthalmol Vis Sci. 2005;46(2):655-662.
- O’Rourke MJ. Ophthalmic Drug Delivery: History, Status, and Trends for the Future. Cataract and Refractive Surgery Today. 2019. https://crstoday.com/articles/2019-june/ophthalmic-drug-delivery-history-status-and-trends-for-the-future/ . Accessed 8/11/2021.
- NDA 211911 Durysta Approval letter, 2020.
- DURYSTA (bimatoprost intracameral implant), for intracameral administration. Allergan USA, Inc. Madison, NJ 07940, 2020.
- Comparison of Bimatoprost SR to Selective Laser Trabeculoplasty in Patients With Open-Angle Glaucoma or Ocular Hypertension. ClinicalTrials.gov . https://clinicaltrials.gov/ct2/show/NCT02507687 . Accessed September 8, 2021.
- Evaluation of the Duration of Effect of Bimatoprost SR in Participants With Open-Angle Glaucoma or Ocular Hypertension. ClinicalTrials.gov . https://www.clinicaltrials.gov/ct2/show/NCT03850782 . Accessed September 8, 2021.
- Medeiros FA, Walters TR, Kolko M, et al. Phase 3, Randomized, 20-Month Study of Bimatoprost Implant in Open-Angle Glaucoma and Ocular Hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627-1641.
- Shirley M. Bimatoprost Implant: First Approval. Drugs Aging. 2020;37(6):457-462.
- Seal JR, Robinson MR, Burke J, et al. Intracameral Sustained-Release Bimatoprost Implant Delivers Bimatoprost to Target Tissues with Reduced Drug Exposure to Off-Target Tissues. J Ocul Pharmacol Ther. 2019;35(1):50-57.
- Craven ER, Walters T, Christie WC, et al. 24-Month Phase I/II Clinical Trial of Bimatoprost Sustained-Release Implant (Bimatoprost SR) in Glaucoma Patients. Drugs. 2020;80(2):167-179.
- Efficacy and Safety Study of Bimatoprost Sustained-Release (SR) in Participants With Open-angle Glaucoma or Ocular Hypertension. ClinicalTrials.gov . https://clinicaltrials.gov/ct2/show/NCT02247804 . Accessed September 8, 2021.
- Efficacy and Safety of Bimatoprost Sustained-Release (SR) in Patients With Open-Angle Glaucoma or Ocular Hypertension. ClinicalTrials.gov . https://clinicaltrials.gov/ct2/show/NCT02250651 . Accessed September 8, 2021.
- Ayala M, Chen E. Predictive factors of success in selective laser trabeculoplasty (SLT) treatment. Clin Ophthalmol. 2011;5:573-576.
- Miller PE, Eaton JS. Medical anti-glaucoma therapy: Beyond the drop. Vet Ophthalmol. 2021;24 Suppl 1:2-15.
- Open Label, Sequential-dose Study of PA5108 Latanoprost FA SR Ocular Implant for Mild-moderate Glaucoma. ClinicalTrials.gov . https://clinicaltrials.gov/ct2/show/NCT04060758 . Accessed September 8, 2021.
- Capitena Young CE, Kahook MY, Seibold LK. Novel Drug Delivery Systems for the Treatment of Glaucoma. Current Ophthalmology Reports. 2019;7(2):143-149.
- Glaukos Investor Presentation. [Available from: http://s21.q4cdn.com/471661912/files/doc_presentations/2019/Glaukos-Presentation_January-2019.pdf . Accessed September 8, 2021.
- NWEyes – Our focus is your vision. https://www.nweyes.com/northwest-eye-clinical-research-update-the-glaukos-idose-gc-012-study/ . Accessed September 8, 2021.
- Ocular Therapeutix. Transforming Drug Delivery. https://ois.net/wp-content/uploads/2019/10/Ocular-Therapeutix.pdf . Accessed September 8, 2021.
- Safety and Efficacy of ENV515 Travoprost Extended Release (XR) in Patients With Bilateral Ocular Hypertension or Primary Open Angle Glaucoma. ClinicalTrials.gov . https://clinicaltrials.gov/ct2/show/NCT02371746 . Accessed September 8, 2021.
- PR Newswire. PolyActiva successfully completes initial clinical trial with Latanoprost FA SR Ocular Implant delivering glaucoma treatment to patients over a six-month period. https://www.prnewswire.com/news-releases/polyactiva-successfully-completes-initial-clinical-trial-with-latanoprost-fa-sr-ocular-implant-delivering-glaucoma-treatment-to-patients-over-a-six-month-period-301166914.html . Accessed September 8, 2021.
COMPOUNDED MEDICATIONS
BY WILLIAM F. WILEY, MD
Compounded drugs are made-to-order combinations of two or more drugs that have already been individually approved for use by the FDA. Compounded drugs serve as an important alternative to branded and generic products — they offer good value for patients, compliance benefits and more choices for physicians.
As a cataract surgeon, I pay close attention to tiny details that can affect my surgical outcomes, such as hand position, the amount of fluid used, the exact axis of astigmatism based on multiple preoperative measurements. So it is frustrating how dependent we are on factors outside of our control when it comes to preventing endophthalmitis and postoperative inflammation. We need patients to use the correct antibiotics and anti-inflammatory drops on an appropriate schedule — and there are a lot of barriers to achieving that goal with commercially available, single-agent drugs.

By giving my cataract patients a compounded sub-Tenon’s triamcinolone/moxifloxacin injection at the end of surgery and a compounded once-daily drop of prednisolone/moxifloxacin/nepafenac for 4 weeks, I am able to regain a measure of control over this essential aspect of surgical success. I ensure that patients actually get what I prescribe, with no pharmacy call-backs, pre-authorization requirements or substitutions. Additionally, because I’m either delivering the drugs intracamerally myself or dispensing a single bottle, I’m much less concerned about patient compliance with a confusing drop regimen.
Compounding also provides the opportunity to give patients preservative-free drops to minimize their exposure to preservatives, help them control the cost of postoperative or chronic medications, customize a drop to their unique needs or provide access to medications that are otherwise in short supply.
It is important to know how your compounded drugs are made. State-regulated 503A facilities are permitted to formulate compounded medications for limited use in individual patient cases.
503B FDA-registered outsourcing facilities like ImprimisRx and Leiters are regulated under the Drug Quality and Security Act. Medications from these 503B facilities are made in U.S. facilities with the same oversight and inspections as major pharmaceutical companies, so doctors can be confident in the drugs they are ordering.
CORNEAL CROSS-LINKING
BY BRANDON D. AYRES, MD
Corneal collagen cross-linking has been a critical development for corneal surgeons and our patients. Long available outside the United States, cross-linking with the iLink platform (Glaukos) was approved by the FDA for use in 2016. Most insurance companies now cover both the drugs (Photrexa Viscous [riboflavin 5’-phosphate in 20% dextran ophthalmic solution] and Photrexa [riboflavin 5’-phosphate ophthalmic solution], Glaukos) and the cross-linking procedure when performed with the iLink platform.
Epi-off cross-linking has been shown to be effective at preserving vision and corneal integrity. The procedure halts progression in 92%-100% of cases.1 Patients treated with cross-linking maintain long-term corneal stability for at least 10 years.2,3 For our patients, cross-linking can help avoid progression to costly specialty lenses and, most importantly, avoid the risks associated with penetrating keratoplasty (PK).4 Historically, up to 21% of patients with progressive keratoconus undergo a PK procedure, with more than half requiring multiple transplants within 20 years.5,6 While corneal transplantation has also improved in recent years, we would still like to avoid this end-stage treatment.
A recent simulation model suggests that cross-linking reduces the rate of PK by 26%, with patients spending 28 fewer years in the advanced stages of keratoconus.4 Cross-linked patients have lower anxiety and better vision- and health-related quality of life after cross-linking.7,8 In my experience, it’s empowering for patients to know they have done everything they could to prevent further progression.
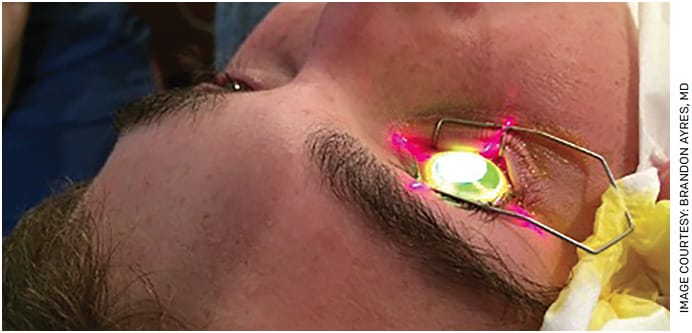
Cross-linking makes financial sense, as well. Compared to conventional treatment, it is associated with lower lifetime medical costs for patients and society and substantial increases in quality-adjusted life years (a measure of economic efficiency), particularly with earlier intervention.4
New developments in cross-linking are on the horizon as well. We are learning more about how to use genetic testing to better guide follow-up of close relatives of keratoconus patients. Additionally, a Phase 3 clinical trial of the iLink system for epi-on cross-linking recently met its primary endpoint of a difference of ≥1.0 D mean change in Kmax between treatment and control arms from baseline to month 6, setting the stage for an epi-on FDA submission next year.
The data on the benefits of cross-linking are clear. Our challenge now is to continue to improve access and to encourage earlier identification and referral of patients with progressive keratoconus.
REFERENCES
- Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg 2009;35:1358–1362.
- Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg 2015; 41:41-46.
- Mazzotta C, Traversi C, Baiocchi S, et al. Corneal collagen cross-linking with riboflavin and ultraviolet A light for pediatric keratoconus: Ten-year results. Cornea 2018;37:560-566.
- Lindstrom RL, Berdahl JP, Donnenfeld ED, et al. Corneal cross-linking versus conventional management for keratoconus: a lifetime economic model. J Med Econ 2020; online ahead of print.
- Pramanik S, Musch DC, Sutphin JE, Farjo AA. Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology 2006;113:1633-1638.
- Maharana PK, Agarwal K, Jhanji V, Vajpayee RB. Deep anterior lamellar keratoplasty for keratoconus: a review. Eye Contact Lens 2014;40:382-389.
- Hersh PS, Stulting RD, Muller D, et al; U.S. Crosslinking Study Group. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology 2017;124:1259-1270.
- Brooks NO, Greenstein S, Fry K, Hersh PS. Patient subjective visual function after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg 2012;38:615-619.
COVID-19
BY ELIZABETH YEU, MD
In the last 18 months, the current epidemic has created a heightened sense of urgency to take care of ourselves and our family. This is omnipresent at many levels of health care — running understaffed due to the health needs of our work family, adjusting to health-care protocols in clinic, interacting with our patients and how we listen and communicate with patients. As one of the most disruptive and biggest developments in ophthalmology in the last 25 years, on a larger scale, practices were forced to shut down “non-urgent” surgeries and experienced clinical slow-downs with layoffs and furloughs. Unemployment and virtual education for students were other larger scale realities and added to the chaos.
From a patient perspective, due to the limited “real estate” behind patients’ ears, between mask loops, hearing aids and spectacle rims, patients are eager sought out options to have some greater independence from their spectacles. As the prolonged nature of the viral pandemic has worn on, it has taken a real mental health and spiritual toll. Patients come in — some afraid and others temperamental about having to wear a mask for the office visit — and they want someone to listen. During the evaluations, patients appear more burdened, and they often want to share with me their very specific reasons as to why they desire refractive or cataract surgery. It is no longer a general, “I want to see better”-type of statement. They have specific motivations, including being able to visit a close family member whom they could not see for over a year or to get their vision straight for a rescheduled or long-delayed vacation.

Through my interactions, patients yearn for active listeners and for doctor who are emotionally tuned in enough to recognize the negative consequences that COVID-19 has had on them specifically. I have seen more frequent second opinions for surgery because patients are acutely sensitive to how insensitive or disconnected their prior doctor may have seemed. Through this, I am learning to be a more patient and empathetic listener, but listening without shouldering their emotional burden is challenging. Empathy or compassion exhaustion are always an occupational hazard, but likely more frequent bi-product and consequence of caring for patients during this jarring and life-altering viral pandemic.
With all this being said, the importance of self-care, self-actualization of the need for mental health care and selfishly putting yourself and family first are lessons that have become ever more important to stay grounded and compassionate with our patients.
DRY EYE DIAGNOSTICS
BY PATTI BARKEY, COE
There are many different estimates on the volume of dry eye disease (DED) patients in need of care, ranging anywhere from 34 to 60 million in the United States alone. Luckily, eyecare practices have been fortunate to gain access to some much needed technology during the last 25 years to help when diagnosing these patients.
DED can encompass multiple factors, and each factor needs to be addressed for the patient to feel more comfortable. Many of these patients have been dismissed in the past by providers and may be skeptical as to whether the many new treatments, products and medications will benefit them. The diagnostics give eyecare providers an opportunity to not only diagnose but to educate the patient and measure treatment results.
At Dry Eye University, we recommend that practices have these diagnostics in place:
- Tear osmolarity testing. Osmolarity testing (TearLab) helps in diagnosing DED and lets the provider know whether the treatment applied is appropriate. Monitoring tear osmolarity at follow-up visits is imperative to help the patient maintain a comfortable ocular surface.
- Gland and surface imaging. Eye-care practices have options in the imaging area, including LipiScan (J&J Vision), Keratograph 5M (Oculus), LipiView II (J&J Vision), Meibox (Box Medical Solutions), LACRYDIAG (Quantel Medical) and VX120+ (Luneau Technology). Gland imaging, surface evaluation, lipid layer and lid closure are all valuable in the diagnosing.
- Inflammation. Quidel’s InflammaDry allows rapid testing in office for MMP9 to allow for treatment of inflammation. This has become a valuable screening for ophthalmic surgical patients preoperatively, before the fitting of contact lenses and the insertion of punctal plugs.
- Questionnaires. There are several questionnaires available for use to help screen patients prior to the examination. One example is SPEED (Standard Patient Evaluation of Eye Dryness), a form that scores a patient’s dry eye as mild, moderate or severe. Utilizing the SPEED form at each encounter allows the patient to see the fluctuation in their metric (score). Korb and Blackie originally created the SPEED form for use with the LipiFlow System (J&J Vision).
- Meibomian gland evaluation. The Korb Meibomian Gland Evaluator (J&J Vision) allows for a repeatable evaluation of gland secretions. Documentation of the gland function is becoming part of the slit lamp evaluation. The KORB evaluator applies the same PSI as a lid blink. Some practices use a simple Q-TIP to evaluate and document secretions.
- Allergy testing. In-office allergy testing helps evaluate the allergy component of DED. This is a simple in-office diagnostic that is invaluable in the diagnostic process. Doctor’s Rx (Bausch & Lomb) and AllerFocus are both easily implemented into eye-care practices.
- Sjogren’s testing. The Sjo test (Bausch + Lomb) is lab-ordered blood work. Severe dry eye patients are often found to be positive for Sjogren’s, which can be a devastating disease. The sooner the patient is diagnosed, the better. Offices that offer Sjogren’s testing often build great referral relationships with local rheumatology practices.
As dry eye care grows in popularity for practices, so does the demand for competition in the diagnostic space. The dry eye space continues to advance with new drugs, new products, treatment options and diagnostics. We look forward to expanding the care for the millions of patients in need with the help of this new technology.
DRY EYE MEDICATIONS
BY PREEYA K. GUPTA, MD
When Ophthalmology Management launched in 1997, dry eye was not even considered a real disease. Treatment was not much more than sending your patient to the pharmacy with instructions to buy artificial tears and use them whenever they felt like their eyes were dry. Tear quality was not a consideration; meibomian gland disease was a wholly separate entity that was not discussed in the context of how tears worked.
This all changed in 2003 with the FDA approval of the first medication to treat dry eye. Restasis (cyclosporine 0.05%, Allergan) was approved after it demonstrated an increase in Schirmer test results when compared with its vehicle. We now know that ocular surface inflammation is a major contributor to dry eye. It took more than 10 years for another dry eye medication to win approval. Xiidra (lifitegrast, Novartis) was brought to market after demonstrating an improvement in both ocular signs associated with inflammation like corneal fluorescein staining. Xiidra also showed an improvement in dry eye symptoms. Restasis and Xiidra have been joined by another cyclosporine-containing medication, Cequa (cyclosporine 0.09%, Sun Ophthalmics), which has novel drug delivery methods to improve ocular surface penetration.
Topical steroids have been utilized off-label to treat dry eye for years. At least two of them have FDA labeling that includes many of the clinical signs we all treat (Lotemax, Bausch + Lomb; Flarex, Eyevance). It wasn’t until 2020 that Eysuvis (Kala) became the first FDA approved topical steroid approved to treat dry eye signs and symptoms.
Medical treatment of meibomian gland disease and blepharitis has been challenging. To date, there are no FDA-approved medications available. Thankfully, this is one of the most active areas of development in the ophthalmic pharmaceutical industry. Companies to watch include Bausch & Lomb, Tarsus and Azura.
From a near non-entity in 1997 to one of the most active areas of research and commercial development in eye care, the medical treatment of dry eye has enjoyed meteoric growth and we will see more to come.
DRY EYE TREATMENT DEVICES
BY ALICE T. EPITROPOULOS, MD, FACS
It was not too long ago that tears were the only treatment available for dry eye disease (DED). This area in ophthalmology has drawn quite a bit of attention over the last few years, mainly because of the expansion of diagnostic technologies and more effective therapies. Furthermore, we’ve come to realize how untreated DED can affect our patients’ quality of life and visual outcomes after cataract or refractive surgery.
Meibomian gland dysfunction (MGD) is thought to be the leading cause of DED. Its effects can be debilitating, with patients often complaining of burning, fluctuations in visual acuity, contact lens intolerance, and it can have a negative impact on our surgical outcomes. Inflammation and MGD have become primary targets for testing and treating this bothersome and ubiquitous disease.
The overall impact of MGD involves a loss of the normal lipid layer of the tear film that can result in an evaporative form of DED. In advanced cases, there can be permanent gland atrophy, scarring and erosion of the posterior lid margin, altered microbial flora of the ocular surface and chronic inflammation.
Revolutionary advances have been made in the diagnosis and management of MGD, leading to several new in-office treatment options for an otherwise complex disease process.
Treatment of the lid margin can be divided into three approaches: lid margin hygiene, removal of obstruction and reduction or elimination of inflammation. These three treatment categories work synergistically and are most effective when performed earlier in the disease process, before patients reach end-stage MGD with significant gland atrophy and dropout.
While at-home options such as lid scrubs, topical immunomodulators, antibiotic/anti-inflammatory and high quality nutritional supplements have been shown to be therapeutic in patients with DED/MGD, the following in-office MGD treatment options have been truly innovative:
- BlephEx (Alcon) uses a medical grade microsponge to remove the biofilm reducing bacteria/demodex that contributes to inflammation and obstruction of the glands. (NuLids [NuSight Medical], another lid hygiene option, is designed for home use.)
- LipiFlow thermal pulsation (J&J Vision), approved in 2011, uses a combination of heat and vectored pulsation applied to the posterior and anterior eyeline to unclog obstructed meibomian glands. A single treatment has the potential to last 2-3 years, according to a study by Greiner published in 2016 in Eye & Contact Lens.
- iLux (Alcon) is a handheld thermal device that allows direct visualization of the glands through a magnifier while heating and expressing the glands via a light source. Treatment can be customized to target certain glands that appear more inflamed or obstructed.
- TearCare (Sight Sciences) is a device that applies heat externally to both lids for 15 minutes; patients can blink normally during the procedure, which is followed by manual expression.
- Mibo Thermoflo (MiBo Medical) delivers heat at 108° F to the tarsal conjunctiva through a non-disposable silver eye pad.
- Intense pulsed light, which has traditionally been used in dermatology for treating pigmented or vascular lesions, has become more popular for patients with MGD; the FDA recently granted De Novo authorization to Lumenis for its device for improving signs of DED due to MGD
- Intraductal probing can be performed using a microcannula to open meibomian gland orifices under local anesthesia.
Considering the high prevalence of MGD as a cause of DED and its consequential effects, early identification and treatment of MGD is critical. We have entered a new era for innovation and treatment of DED and MGD, with promising and novel therapies. Incorporating these advanced office-based treatments is allowing for greater success in management of the disease and preventing atrophy to the glands, thereby improving long-term patient outcome.
ELECTRONIC MEDICAL RECORDS
BY ELLIOT LEVINE, MD
The evolution of electronic medical records (EMR) dates back to the 1960s when Lockheed developed an electronic clinical information system. The ‘70s saw companies begin to develop EMRs for hospitals and universities. The Department of Veterans Affairs began using an EMR initially called DHCP, which later became known as Vista A.
Since then, we have seen the development of ophthalmology-specific EMR that have allowed us to organize pertinent data, draw pictures and view our imaging device results all within one electronic system. Now, we have the ability to integrate optical, contact lens and ASC utilities into one product.
Many systems now come with an integrated enterprise practice management, or EPM. Add to that the ability to access it all through cloud-based systems, and you have the modern day ophthalmology EMR.
Five years ago, our practice began the process of converting from paper charts to electronic charts. We are a multisubspecialty, multilocation practice with over 250,000 paper records. The task was daunting and one of the reasons we were late adopters of EMR.
Our conversion process was slow, methodical and deliberate. It was an eye-opening experience as it caused us to look at every workflow that went into running our practice. It was a journey that ultimately led us to a higher level of compliance, efficiency and level of care. This in turn will make us more competitive in our market and financially sustainable.
Our EMR has become the foundation from which all aspects of running our practice emanate. As technology advances and medical practices adopt these technologies, our processes will become more streamlined and less vulnerable to errors. We are now seeing this with integrations of our diagnostic and therapeutic devices, practice management system and engagement with our patients.
The seamless transfer of data frees up our staff to become more productive, reduce errors and better able to interact with our patients in a more professional and timely manner, thus allowing us to provide better care to our patients. A good example of this is how EMRs send data to the IRIS registry. Additionally, the transfers of data from our biometers to our microscopes allow for more accurate alignment of toric lenses.
I once was a skeptic, but now I am a true believer in the promise of EMR. There is no going back, and embracing the future of medicine with an open mind, patience and putting the patient first is the recipe for success.
ENDOTHELIAL KERATOPLASTY
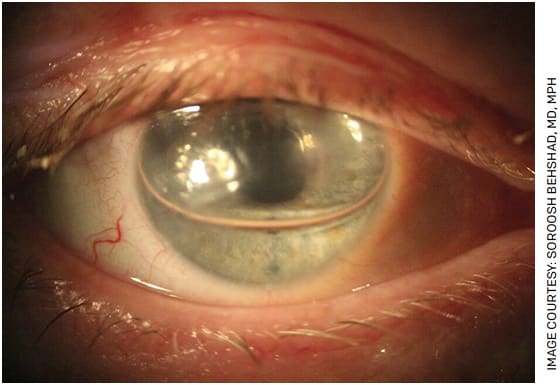
BY SOROOSH BEHSHAD, MD, MPH
With more than two decades since it was first described,1 endothelial keratoplasty (EK) has completely revolutionized the treatment of vision loss caused by corneal endothelial pathology.
Although more challenging compared to previous full-thickness penetrating keratoplasty (PK), the EK surgical techniques such as Descemet stripping endothelial keratoplasty (DSEK) and Descemet membrane endothelial keratoplasty (DMEK) have allowed for smaller incisions, greater safety, quicker recovery and better visual outcomes.2 These key advantages have decreased the threshold for surgical intervention for corneal endothelial pathology when compared to conventional PK.
Since the early days of DSEK, we have seen an evolution to thinner grafts and less cumbersome donor tissue preparation techniques, which were greatly advanced with the introduction of the microkeratome. The ultimate goal of thinner grafts and quicker visual recovery was greatly advanced again when DMEK was first described in 2006. Compared to the adoption of DSEK from PK, DSEK to DMEK adoption has been slower, which is felt to be due to more difficult tissue preparation and a steeper learning curve for surgeons with this surgical technique.3,4
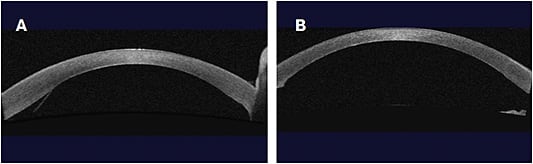
On the international stage, utilization and adaptation of keratoplasty techniques have been restricted due to a shortage of donor cornea tissue. Over the past decade, EK has made an impact by not only allowing for more targeted treatments of corneal pathology but also allowing for use of one corneal donor tissue to be utilized for multiple recipients. For instance, the endothelium from one donor can be used for EK, while the stroma from the same donor can be utilized for deep anterior lamellar keratoplasty for another recipient. This has been effective and impactful in low-resource settings of the developing world.
Over the past 15 years, EK cases have grown to surpass PK cases. DSEK cases peaked around 2013, and the number of DMEK cases completed by US surgeons continues to increase each year.5 In the future, we plan to see EK moving towards further development and improvement in the areas of endothelial graft survival, surgical techniques/tissue delivery and overall improvement of visual predictability. Even with the potential introduction of biologic and pharmacologic treatments for endothelial pathology coming down the pipeline, EK is a surgical technique that is likely here to stay.
REFERENCES
- Melles GR, Eggink FA, Lander F, et al. A surgical technique for posterior lamellar keratoplasty. Cornea. 1998;17:618-626.
- Price MO, Feng MT, Price FW Jr. Endothelial Keratoplasty Update 2020. Cornea. 2021;40(5):541-547.
- Melles GR, Ong TS, Ververs B, et al. Descemet membrane endothelial keratoplasty (DMEK). Cornea. 2006;25:987-990.
- Tenkman LR, Price FW, Price MO. Descemet membrane endothelial keratoplasty donor preparation: navigating challenges and improving efficiency. Cornea. 2014;33:319-325.
- Eye Bank Association of America. 2019 eye banking statistical report. Washington, DC: Eye Bank Association of America. 2020. Available at: https://restoresight.org/whatwe-do/publications/statistical-report/ . Accessed August 30, 2021.
FEMTOSECOND LASER-ASSISTED CATARACT SURGERY
BY KENDALL E. DONALDSON, MD, MS
It has now been a decade since the femtosecond laser was approved for use in cataract surgery, initiating the term FLACS (femtosecond laser-assisted cataract surgery). It’s been a decade of controversy, with surgeons polarized on the topic. Yet, FLACS offers undeniable benefits, including creation of a perfect capsulotomy of a precise size, shape and location.1
Research demonstrates that the laser can serve as a tool to help us use less phacoemulsification energy during surgery, making our surgery less traumatic.2 Additional data show that endothelial cells may be less traumatized during surgery when the laser is used to facilitate pre-fragmentation of the lens material.3 Clearly, these benefits are coveted, as other technology (such as Zepto [Centricity] and MiLoop [Zeiss]) have attempted to duplicate the effects with a more financially feasible model.
FLACS has also encouraged us to address small amounts of residual astigmatism during cataract surgery by giving surgeons the confidence to perform simple, accurate limbal relaxing incisions (LRIs). Prior to 2011, little attention was paid toward residual astigmatism.
Several large studies have been performed internationally showing no differences in refractive results or safety when FLACS was compared to traditional cataract surgery.4,5 Despite these large studies, several smaller studies have shown FLACS to be a helpful tool in complex cases, such as Fuchs’ dystrophy, traumatic cataracts, loose zonules, pseudoexfoliation syndrome, shallow anterior chamber and mature cataracts.6 In addition, LRIs have been reintroduced into clinical practice, which has been a very helpful adjunct to premium lens surgery (which became more popularized as FLACS increased in usage).7
The femtosecond laser is a useful tool for cataract surgeons, although penetration remains limited. FLACS requires both a financial commitment and a logistical commitment from a practice, which many practices have found difficult to make. However, the barrier to entry is easing through the years. I look forward to additional research and further integration of FLACS with other advancing technologies in cataract surgery.
REFERENCES
- Friedman NJ, Palanker DV, Schuele G, et al. Femtosecond laser capsulotomy. J Cataract Refract Surgery. 2011 Jul;37:1189-1198.
- Conrad-Hengerer I, Hengerer FH, Schultz T, Dick B. Effect of femtosecond laser fragmentation on effective phacoemulsification time in cataract surgery. J Refract Surg. 2012 Dec;28:879-83.
- Fan W, Yan H, Zhang G. Femtosecond laser-assisted cataract surgery in Fuchs’ endothelial corneal dystrophy: Long-term outcomes. J Cataract Refract Surgery. 2018; July 44:864-870.
- Lundström M, Dickman M, Henry Y, et al. European Society of Cataract and Refractive Surgeons. Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: study from the European Registry of Quality Outcomes for Cataract and Refractive Surgery. Femtosecond Laser–Assisted Cataract Surgery Study Collaborators. J Cataract Refract Surg. 2017 Dec; 43:1549-1556.
- Schweitzer C, Brezin A, Cochener B, et al; FEMCAT study group. Femtosecond laser-assisted versus phacoemulsification cataract surgery (FEMCAT): a multicenter participant-masked randomized superiority and cost-effectiveness trial. Lancet. 2020 Jan 18;395(10219):212-224.
- Fan W, Yan H, Zhang G. Femtosecond laser-assisted cataract surgery in Fuchs endothelial corneal dystrophy: Long-term outcomes. J Cataract Refract Surg. 2018 Jul;44:864-870.
- Roberts HW, Wagh VK, Sullivan DL, Archer TJ, O’Brart DPS. Refractive outcomes after limbal relaxing incisions or femtosecond laser arcuate keratotomy to manage corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2018 Aug;44:955-963.
FEMTOSECOND LASER LASIK FLAPS
BY GEORGE O. WARING IV, MD, FACS
A femtosecond is approximately one millionth of one billionth of one second, and this fraction of time has revolutionized modern day lamellar laser vision correction. Since the original mechanical keratomes described by Barraquer, LASIK has evolved into perhaps the single best studied elective procedure in the human body.
The first start-up company to commercialize femtosecond laser technology for ophthalmology was formed in Ann Arbor, Michigan, in 1997 by Ronald Kurtz, MD, and Tibor Juhasz, PhD. A year later, the first clinical prototypes were finalized. On Dec. 10, 1998, the first human femtosecond laser procedure was performed in Budapest, Hungary, by Imola Ratkay Traub, MD, PhD. In November 2000, the first commercial femtosecond laser for corneal surgery was introduced at the annual AAO meeting in Dallas, and the first commercial sale occurred a year later.1
The early days of femtosecond lasers for lamellar corneal flap creation were less than ideal. Long treatment times, severe opaque bubble layer requiring long clearing times, postoperative inflammation and other phenomenon unique to femtosecond lasers were the norm. However, pioneers such as Stephen Slade, MD, Daniel Durrie, MD, Lee Nordan, MD, Jon Dishler, MD, FACS, and others could see the future. Thanks to these early efforts, this enabling technology evolved, creating more precise and predictable outcomes and increased efficiencies with better surgeon and patient experiences. Although it took time, the body of evidence mounted supporting non-inferiority and slowly transitioned to superiority in many facets of LASIK flaps relative to a manual keratome. Fast forward to today: US refractive surgical procedures have grown at an enormous pace, five femtosecond lasers are on the market for LASIK flap creation and the vast majority of lamellar LASIK flaps are created with femtosecond lasers.
Further iterations on the horizon promise higher repetition rates, smaller form factors, combined technologies and small incision lenticule creation.
The femtosecond laser has benefited us as surgeons and our patients, changing laser vision correction forever.

REFERENCE
- Waring IV GO, Rocha KM. Femtosecond lasers in Cornea and Lens Surgery. First ed. Slack inc.
IOL FORMULA ADVANCES
BY UDAY DEVGAN, MD, FACS
When Ophthalmology Management started 25 years ago, cataract surgery was primarily thought of as a procedure to clear the visual axis and perhaps address some of the refractive error of the eye. We have come a long way since then. Now, cataract surgery is the most commonly performed refractive surgical procedure in all of ophthalmology. It is also the most powerful, with the ability to treat severe hyperopia, extreme myopia, high degrees of astigmatism, presbyopia and everything in between.
To deliver great refractive outcomes with cataract surgery, we need to have accurate biometry and precise IOL calculations. Biometry has advanced over the years, with the biggest jump being the move from ultrasound-based A-scan to optical coherence biometry, a technology that brings a much higher degree of precision and repeatability. Now that we can accurately measure the axial length and anterior chamber depth of the eye to within 0.01 mm and the keratometry to a small fraction of a diopter, why are current formulae only about 80% accurate in delivering the target refraction +/- 0.5 diopters?
The IOL calculation formulae have evolved dramatically since the 1970s when the concept started gaining traction. The early regression formulae were then modified with adjustments before being discarded for theoretical formulae that needed just the keratometry and axial length to determine the effective lens position. The most commonly used formulae now include anterior chamber depth (ACD) to increase precision over prediction of the final resting position of the IOL.
Many other factors play roles in the accuracy of IOL calculations, including posterior cornea, equatorial lens position, age, aphakic refraction, relative ratio of various eye segments, specific IOL parameters and even surgical technique. These variables do not occur in a vacuum and are likely intimately related to other variables such as ACD.
The limiting factor in IOL calculation accuracy is not biometry or even creating another static formula. This is where artificial intelligence, machine learning and a big data approach can help improve accuracy to 90% or even 95%. AI will not replace physicians; however, physicians who use AI will replace physicians who don’t.
MINIMALLY INVASIVE GLAUCOMA SURGERY
BY STEVEN R. SARKISIAN, JR., MD
Of all the subspecialties in ophthalmology, it could be said that glaucoma has seen the most disruptive change over the last 25 years. Glaucoma has shifted from a disease treated primarily with pharmaceuticals to a disease treated surgically. It is the MIGS — for minimally invasive glaucoma surgery — revolution that has initiated this change.
In 1992, Martin Uram, MD, MPH, developed endoscopic cyclophotocoagulation (ECP) and it became adopted by general ophthalmologists, glaucoma specialists and even retinal specialists around the world to treat glaucoma at the time of cataract surgery or for refractory glaucoma. In many ways, ECP was the first MIGS procedure because it was bleb-free and could be used to treat all stages of glaucoma and multiple types of glaucoma, including angle-closure glaucoma. Then in the early 2000s, George Baerveldt, MD, developed the Trabectome procedure (MST), which was used to remove a strip of trabecular meshwork (TM) with a bipolar electrode under direct gonioscopic view. The first Trabectome procedure in the United States was in 2006.
The 2010s were, in many ways, the decade of MIGS. In 2012, the FDA approved the iStent (Glaukos) to bypass the TM, followed by the iStent inject and the Hydrus implant (Ivantis) in 2018. (The CyPass micro-stent [Alcon] was FDA approved in 2016, but was withdrawn from the market 2 years later due to concerns with endothelial cell loss.) The last decade also saw the increase in ab interno canaloplasty and goniotomy with a variety of devices, such as the OMNI Surgical System (Sight Sciences) and its predicate devices the TRAB360 and VISCO360, the Kahook Dual Blade (New World Medical) and the Trab-Ex goniotomy system (MST).
Also, we saw the resurrection of the iTrack microcatheter, a device intended for ab externo canaloplasty, and repurposed as an ab interno technique to perform 360° of canal viscodilation.
In addition, Allergan released the XEN Gel Stent. Although not a true MIGS procedure because it is bleb-forming, it is certainly a less-invasive procedure, especially with clinically significant hypotony being truly rare.
Other new surgical MIGS devices are currently in clinical trials. Combined with the advent of sustained-release implant of drugs, these should lead to the full manifestation of the interventional glaucoma revolution and the potential for the near-cessation of eyedrops to treat glaucoma.
OPTICAL BIOMETRY
BY WILLIAM TRATTLER, MD
The development of the optical biometry, which Zeiss released in September 1999, changed the landscape of cataract surgery. This technology, which utilizes partial coherence interferometry, replaced ultrasound as a method for measuring the axial length of eyes. Axial length measurements are one of the key values used in IOL formulas to calculate the optimal power of the IOL. Having a more accurate measurement of axial length allowed for more accurate IOL planning, which resulted in a dramatic improvement in visual outcomes for patients undergoing cataract surgery.
Optical biometers are also able to streamline axial length measurements, as the device acquires axial length measurements faster and easier than ultrasound. Further, optical biometers are able to measure other parts of the anterior segment of the eye, including corneal steepness, corneal diameter (white to white), lens thickness and anterior chamber depth. These additional measurements were then incorporated into updated IOL formulas, such as the Holladay 1, Hoffer Q and SRK/T. Over the years, the availability of reliable and repeatable measurements of the structures of the eye allowed for further improvements in IOL formulas, such as the Holladay 2, Haigis, Olsen, Hill RBF and the Barrett formulas.
Innovations in optical biometry have continued. The latest generation of optical biometers use OCT to measure the structures of the eye. Both the Argos (Santec/Alcon) and IOL Master 700 (Zeiss) are widely available. Incorporating OCT has helped improve the ability of optical biometers to measure the axial length in patients with very advanced cataracts, as demonstrated by Shammas et al in 2016.
In summary, the development of optical biometry for measuring the eye, along with the development of more advanced IOL formulas, have benefited patients with improved visual outcomes. These improvements also allowed for the development of premium IOLs, such as toric, accommodating, extended depth of focus and multifocal IOLs, which all require accurate power selection. Other innovations continue to improve visual outcomes with cataract surgery, but the development of optical biometry can clearly be considered one of the most significant developments in ophthalmology during the past 25 years.
OPTICAL COHERENCE TOMOGRAPHY
BY DAVID HUANG, MD, PHD
As a co-inventor of optical coherence tomography (OCT), it is gratifying to see the introduction and wide adoption of this technology over the past 25 years. The first commercial product, OCT1 (Humphrey Instruments, now a division of Zeiss), was introduced in 1995. Initially, many clinicians were dubious that the grainy retinal cross-sections had any usefulness over clinical examination.
The much-improved Stratus OCT was introduced in 2002, and by then OCT had surpassed fundus photography and fluorescein angiography to become the most commonly billed ophthalmic imaging procedure, according to CMS. By 2006, several companies entered the market with Fourier-domain OCT systems, which took speed and resolution to an even higher level and made OCT ubiquitous in ophthalmology clinics.
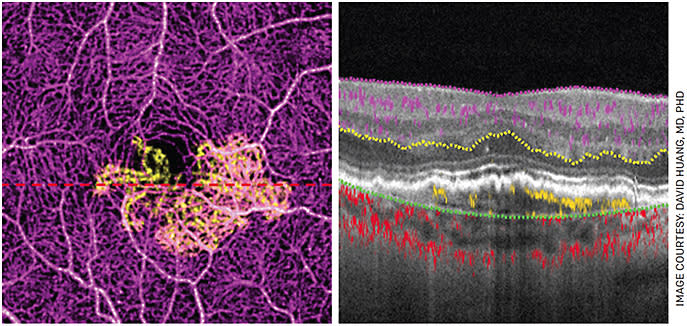
Many drivers contribute to the success of OCT. From the beginning, it was obvious that OCT is the only modality with sufficient axial resolution to visualize retinal layers. Over time, the speed has increased from 100 Hz (axial scans per second) to the current 200 kHz for the fastest commercially available clinical system, doubling every 2.4 years. By 2014, speed was sufficient to make OCT angiography (OCTA) clinically practical.
The use of tracking and averaging of OCT scans to suppress speckle also greatly improved image quality. The introduction of sight-saving anti-VEGF injections for wet AMD and diabetic macular edema made OCT necessary for almost every visit to the retina clinic. This versatile instrument is also commonly used for anterior segment and optic nerve evaluation. According to the AAO IRIS Registry, it is now used more frequently than visual field to monitor glaucoma.
OCTA is the most important innovation in OCT in the past decade. It uses motion contrast to provide 3D visualization of blood flow down to the capillary level. Because no dye injection is needed, OCTA can be used for routine screening and monitoring. OCTA is used to detect neovascularization and capillary loss in AMD, diabetic retinopathy and many other diseases. Most new OCT devices now offer OCTA capability, and I foresee rapid adoption.
PRESBYOPIA-CORRECTING IOLS
BY JOHN A. HOVANESIAN, MD
As we celebrate the 25th anniversary of Ophthalmology Management, we are also soon to celebrate the 25th anniversary of the FDA approval of the first presbyopia-correcting IOL (PC-IOL): the Array implant by AMO (now Johnson & Johnson Vision) launched in the United States in 1997. We have since had an expanding and ever-improving armamentarium of presbyopia-correcting implants with some highly impressive multifocal designs to offer patients.
I put PC-IOLs into two categories: those that offer the highest spectacle independence but come at a cost of some glare and halos, and those with a more favorable side effect profile but a more limited range of vision.
A recent study I published with Quentin Allen, MD, and Michael Jones, MD, examined patient reported outcomes of the AcrySof IQ PanOptix trifocal from Alcon, and 83% of patients reported they never needed glasses for any activity at any distance — twice as high as any implant we have ever studied. Glare and halo incidence and severity were similar to other current multifocals. Johnson & Johnson Vision’s new Synergy implant, another trifocal, promises similar results.
In the second category of implants — causing less side effects but with more limited range of vision — stands Alcon’s AcrySof IQ Vivity lens. This non-refractive multifocal offers about a 1.5-D range of vision. Targeted for emmetropia or 0.25 D, most patients have very comfortable distance and intermediate vision. According to another study I conducted with the same co-authors, approximately 50% of patients can also read while “never” or “rarely” wearing reading glasses. Most importantly, this lens has been shown repeatedly to cause no more glare and halos than a monofocal IOL. My experience with hundreds of eyes now affirms this claim.
Some surgeons enthusiastically recommend implanting a mix of a non-diffractive Vivity in the dominant eye with a trifocal (PanOptix or Synergy) in the fellow eye to achieve high spectacle independence and patient acceptance.
For me, bilateral implantation of these lenses, typically with a plano target in both eyes, covers the wide range of patients I see — from those with near perfect eyes to those with comorbidities who might not accept the side effects of a traditional multifocal.
PUPIL TRACKING AND REGISTRATION SYSTEMS
BY ERIC DONNENFELD, MD
Sometimes it is difficult to fathom that the excimer laser trials I participated in occurred more than 30 years ago. Laser treatments were effective but rudimentary, with oblate ablations and minimal blend zones, and we manually tracked the patient’s pupil — the surgeon literally held the patient’s head and aimed at the patient’s pupil. There was no control over eye movement, and ablations were imprecise and sometimes decentered compared to what we have today.
The FDA trials for the first laser approved showed 2-year results, published in 1997, in which 66.5% of patients achieved 20/20 UCVA, and 92.5% had 20/40 or better UCVA. Many patients (6.9%) lost two more lines of BCVA. The recent results from the FDA’s PROWL 1 and 2 studies showed that 99% and 96% of subjects achieved 20/20 bilateral UCVA with no enhancements needed, and 0.1% of patients lost two lines of BCVA.
This remarkable improvement in laser vision correction results is overwhelmingly due to pupil tracking and registration systems.
The first major improvement in excimer laser delivery was pupil tracking. The original tracking system consisted of an infrared laser, a position sensor and adjusting tracking mirrors. The infrared laser emitted a beam that reflected off adjusting tracker mirrors to the pupillary boundary. Light reflected off the pupillary boundary was detected by a position sensor, measuring the magnitude, velocity and rate of acceleration and direction of the eye’s movement.
During surgery, the infrared laser beam automatically acquired and optimized itself to the pupillary boundary of the patient 4,000 times each second, locking and coupling itself to the eye’s saccadic movements.
A drawback of this early system is that it required pupillary dilation and only tracked in the horizontal meridian. An incremental improvement was a tracker that did not require pupillary dilation — it not only tracked in the horizontal plane, but also tracked motion in the Z axis towards and away from the laser, turning off the laser when excessive motion was detected.
The most recent advance in excimer laser delivery is registration that can be applied to both wavefront and topographic ablations. As I showed in my own 2004 study, the pupil is a moving target and centration varies greatly with constriction.3 In addition, the mapping that is performed preoperatively is done with the patient sitting up, while the treatment is done with the patient lying down. This results in cyclotorsion, which again will diminish the accurate application of laser energy.
The solution has been registration. The goal of achieving UCVA following excimer laser ablation that is superior to the BCVA prior to surgery is based on treating the irregularities of the visual system that cannot be corrected with glasses. This has been made possible with pupil tracking and registration.
REFERENCES
- Hersh PS, Stulting RD, Steinert RF, et al. Results of phase III excimer laser photorefractive keratectomy for myopia. The Summit PRK Study Group. Ophthalmology. 1997;104(10):1535-1553. doi:10.1016/s0161-6420(97)30073-30076.
- Eydelman M, Hilmantel G, Tarver ME, et al. Symptoms and Satisfaction of Patients in the Patient-Reported Outcomes With Laser In Situ Keratomileusis (PROWL) Studies. JAMA Ophthalmol. 2017;135(1):13-22.
- Donnenfeld E. The pupil is a moving target: centration, repeatability, and registration. J Refract Surg. 2004;20(5):S593-S596.
ROUTINE SURGERY FOR EPIRETINAL MEMBRANES
BY DANIEL F. KIERNAN, MD, FACS
Epiretinal membrane (ERM) was first described in 1865 as an avascular, fibrocellular membrane that proliferates on the inner retina. ERMs may be idiopathic, related to a posterior vitreous detachment or secondary to causes including surgery, trauma and retinal vascular diseases.1 Although the diagnosis is often incidental to routine retinal imaging with spectral domain optical coherence tomography, ERM increases in prevalence with age and may lead to visual impairment, necessitating intervention to halt progression of symptoms; these include reduced visual acuity, metamorphopsia, loss of stereopsis and aniseikonia.1,2
Numerous innovations have led to the routine surgical treatment of this condition, also termed macular pucker, epimacular proliferation, preretinal macular fibrosis, epiretinal fibrosis/gliosis, surface wrinkling retinopathy and cellophane maculopathy.3
The logistics of vitreoretinal surgery have changed in the last 15 years, with retinal surgeons transitioning from longer general intubation/laryngeal-mask-airway anesthesia to a shorter, local regional block with monitored anesthesia care option and cost-effectively utilizing outpatient ASCs, rather than less efficient and versatile community hospital settings.4 Furthermore, the development of sutureless, self-sealing microincision vitrectomy surgery (MIVS) wound construction, high-speed vitrectomy cutters and instrumentation optimized for retinal microsurgery have given us reductions in operative time and surgical complications.
Spanning more than half a decade, from the first 17-gauge vitrectomy surgery performed by Robert Machemer in 1970 — with the first membrane peel performed by him using a bent-tipped needle — the evolution of retinal surgery has culminated in modern MIVS 23- through 27-gauge instruments, making ERM removal one of the most common indications for posterior segment surgery.5,6 ERM combined with internal limiting membrane peeling and gas tamponade also dramatically improved the closure rate of macular holes after initial descriptions using vitrectomy by Kelly and Wendel in 1991.7-9
Further innovations included a dull-pointed pic by Conor O’Malley, diamond-dusted membrane scraper by Yasuo Tano and diamond-coated/conformal forceps, angled scissors and end-grasping forceps by Steve Charles, as well as chromovitrectomy using vital dyes by Kazuaki Kadonosono.6-9
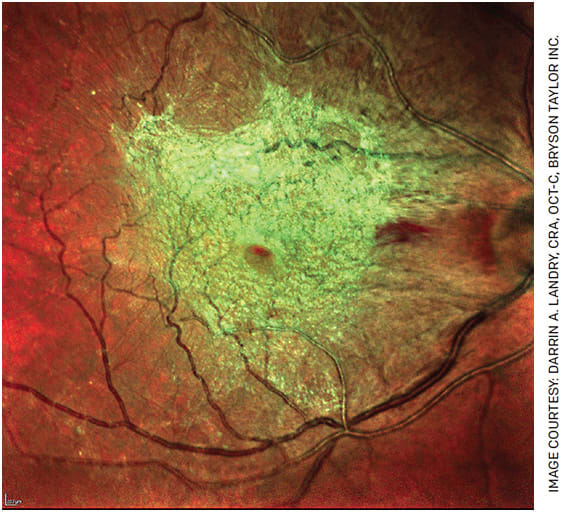
These advances have improved surgeons’ ability to visualize and safely attack even the most gnarly of membranes, successfully eliminate the occupying retinal tractional forces and, to quote General Bernard Montgomery speaking to his troops on the eve of D-Day, “... strike a blow for freedom!” Every time you peel a membrane in the future, I challenge you to sort out how this quote became related to our subspecialty.
REFERENCES
- Fung AT, Galvin J, Tran T. Epiretinal membrane: A review. Clin Experiment Ophthalmol. 2021; 49:289-308.
- Ting FSM, Kwok AKH. Treatment of epiretinal membrane: an update. Hong Kong Med J . 2005;11:496-502.
- Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104:1033-1040.
- Neu L. How We Added Retina to Our ASC. Outpatient Surg Mag. 2007. https://www.aorn.org/outpatient-surgery/articles/outpatient-surgery-magazine/2003/august/retina-asc , accessed Sep 6, 2021.
- Scott MN, Weng CY. The evolution of pars plana vitrectomy to 27-G microincision vitrectomy surgery. Int Ophthalmol Clin. 2016 Fall;56:97-111.
- Charles, Steve (September 2008). “The History of Vitrectomy: Innovation and Evolution”. Retina Today. https://retinatoday.com/articles/2008-sept/0908_05-php . Accessed Sep 6, 2021.
- Kadonosono K, Itoh N, Uchio E, et al. Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol. 2000;118:1116-1118.
- Kusuhara S, Negi A. Predicting visual outcome following surgery for idiopathic macular holes. Ophthalmologica. 2014;231(3):125-32.
- Enaida H, Hisatomi T, Nakao S, et al. Chromovitrectomy and vital dyes. Dev Ophthalmol. 2014;54:120-125.
SELECTIVE LASER TRABECULOPLASTY
BY NATHAN M. RADCLIFFE, MD
As Ophthalmology Management reaches its 25th year, it is appropriate to take a look at one of glaucoma’s most successful treatments of a similar age: selective laser trabeculoplasty (SLT). Developed by Mark Latina, MD, SLT was issued its patent in 1996, 25 years ago. A re-imagination of argon laser trabeculoplasty (ALT), which coagulated tissue and left visible scarring in the angle, SLT was designed to specifically target and excite melanin containing cells in the meshwork. Melanin is a chromophore in the trabecular meshwork, and exciting this pigment with a Q-switched, 532-nm doubled YAG laser induces a biologic response without creating tissue damage. The principle of SLT described above was based by Dr. Latina on the principle of selective photothermolysis, developed in the 1980s by Rox Anderson, MD, and John Parrish, MD.
As for the commercial laser, the company Coherent obtained an exclusive license to the SLT patent from Massachusetts General Hospital in 1996 and later became Lumenis.
At the time SLT launched in the United States after FDA approval in 2001, ALT was fading in popularity, particularly as better therapies such as alpha agonists and prostaglandin analogues were entering the market.
Like most new therapies, physicians did not find the proper place for SLT in the treatment algorithm for many years. Indeed, in its 1998 clinical trial for the FDA, only eyes on maximum medical therapy and eyes on max therapy that had failed ALT were studied. Mistaking this remarkably safe laser therapy as a last-ditch effort, SLT was initially used by most physicians as a second-, third- or fourth-line treatment. Even as safety and efficacy literature surrounding SLT grew, it seems doctors were caught in an ALT algorithm and did not use the laser as a first-line therapy.
Slowly, progress was made, and L. Jay Katz, MD, of the Wills Eye Institute published a primary SLT randomized trial in 2012, paving the way for England’s LiGHT study. The LiGHT study was a multicenter prospective randomized clinical trial. Published in 2018, it demonstrated SLT to be superior to topical therapy such as latanoprost in a multitude of aspects in glaucoma care.
In summary, SLT has come a long way and has improved the quality of life for many patients, earning its current place as a first-line glaucoma therapy. What a difference 25 years can make!
TOPICAL NSAIDs
BY CYNTHIA MATOSSIAN, MD, FACS
We’ve long appreciated the anti-inflammatory benefits of cortisone. As Schalnus established, since the middle of the last century, we’ve witnessed the evolution of steroids and, later, nonsteroidal anti-inflammatory drugs (NSAIDs) in ophthalmology. The first FDA approval for a topical NSAID was Ocufen (flurbiprofen sodium 0.03%, Allergan) in 1986, followed by Voltaren (diclofenac sodium 0.1%, Novartis Ophthalmics) in 1991. Both approvals were for use following ocular surgery. But in 1992, NSAID use broadened with the FDA approval of Acular (ketorolac tromethamine 0.5%, Allergan) for the treatment of seasonal allergic conjunctivitis.
Since then, we now have several commercially available NSAID options at our disposal. However, there were some hurdles along the way that NSAIDs, as a class, had to overcome. Most notably, NSAIDs were the subject of a fair amount of scrutiny when, in 1999, ASCRS first reported that generic formulations were likely causing corneal melts. Our awareness of these effects inspired rigorous study and innovation, bringing about new formulations that limit untoward side effects. Today, NSAIDs are considered an essential pharmacological tool for the treatment of a broad spectrum of ocular conditions.
We now rely on NSAIDs to manage — and even prevent — ocular inflammation and pain following cataract surgery as well as other ophthalmic procedures. Conditions such as cystoid macular edema and maintenance of mydriasis during cataract surgery are likewise more easily managed thanks to NSAID therapy.
This category of medication is also used to alleviate discomfort following corneal refractive procedures. And, as mentioned above, the use of NSAIDs in ophthalmology is not limited to the surgical suite. They continue to also play a role in the treatment of allergic conjunctivitis by providing effective analgesia and relief for mild to moderate pruritus.
NSAID regimens also have become significantly simpler, from four times a day to once a day dosing, decreasing the drop burden for patients and their caregivers. Clinicians can select topical agents with different delivery mechanisms that allow for fast absorption and low residence time, combination drops to reduce therapeutic burden and even NSAIDs that can be added to the irrigation bottle utilized during cataract surgery. Each of these options has a place and is worthy of consideration.
The incorporation of NSAIDs into our field has been one of the primary drivers of progress in ophthalmology. A topical approach to pain control as well as decreased incidence of cystoid macular edema play important roles in the lives of our patients. Thanks to the NSAID options that are available to us today, we can meet the needs of our patients safely and effectively.
TORIC IOLS
BY LISA K. FEULNER, MD, PHD
There was a time when patients were just happy to have ‘”the fog lifted” after cataract surgery, regardless of whether they later needed correction to see clearly. However, patients’ demand for a “perfect” refractive outcome continues to grow with each passing year, and the bar to reach the ideal refractive goal continues to rise. Nearly 25 years ago, in 1998, the first available toric IOL — by STAAR Surgical — was developed, and the quest to correct significant astigmatism with an IOL began.
According to the Eye Diseases Prevalence Research Group in 2004, approximately two-thirds of cataract patients have >0.75 D of astigmatism, and it is generally agreed that residual astigmatism >0.5 D is significant. For many years, the use of toric IOLs was challenging due to variability in pre-operative measurements, poor IOL stability and limited IOL powers. Many of us struggled through these limitations, believing that correcting astigmatism was essential for providing great refractive outcomes for our surgical patients.
Today, we have a better understanding of the importance of considering posterior corneal astigmatism and the impact of the ocular surface. Improvement in the accuracy of measurements, a wider range of available dioptric IOL powers, enhanced toric calculators, improved IOL stability and the use of intraoperative aberrometry has empowered surgeons to use toric IOLs with confidence. The development of toric presbyopia-correcting IOLs has enabled us to offer a broader range of post-operative refractive options to more patients who otherwise might have been excluded based on their astigmatism.
The evolution of preoperative measurements, IOL technology and tools to improve the accuracy of intraoperative toric IOL placement has opened the door for many ophthalmologists who might have previously been hesitant to incorporate “premium lenses” into their practice. As we look to the future, we see newer technologies are already within reach, and even more are on the horizon to expand our ability to correct astigmatism during cataract surgery.
As the bar rises for the ideal post-surgical refractive outcome, surgeons and industry will continue to pursue better ways to answer the call.
WAVEFRONT IMAGING
BY SCOTT M. MACRAE, MD
In the late 1990s, wavefront sensing (or imaging) sparked three exciting revolutions in ophthalmic optics: retinal imaging, the concept of supervision and customized corneal ablation. The ability to use wavefront sensing in conjunction with using a deformable mirror to correct or induce aberrations was an idea borrowed from ground-based astronomy and the world’s largest telescopes, like the Keck Observatory telescopes on top of Maunakea in Hawaii. Using wavefront sensing in 1997, Dave Williams and his visual science group were able to measure a subject’s aberrations and then correct all the eye’s aberrations or add aberrations with a deformable mirror and simulate “super vision” as well as visualize individual cones and rods in live subject with amazing detail.
LASIK surgeons and the industry were quick to recognize the tremendous potential of wavefront sensing to detect all the aberrations of the eye. They developed the new tools needed to participate in one of the greatest revolutions in ophthalmic optics since Ben Franklin invented the bifocal more than 200 years ago.
Since the emergence of wavefront, clinicians have had to learn a new language and gain a deeper understanding of advanced optics with terms such as “lenslets,” Zernike polynomials such as “vertical coma” or “secondary astigmatism” and “wavefront-guided” and “wavefront-optimized.”
Wavefront imaging quickly developed into a powerful tool that allows clinicians to better understand and formulate treatment strategies for keratoconus, ectasia, penetrating keratoplasty and astigmatism management. Because of the wavefront revolution, LASIK technologies quickly evolved from broad-beam lasers to smaller scanning spot lasers and faster, more precise eye-tracking systems. The wavefront sensors helped us understand our patients’ complaints related to decentration, central islands and small optical zones and then design appropriate systems to treat them.
As our understanding of wavefront becomes more refined, it will be critical in evaluating the next generation of premium and extended depth of focus IOLs. Wavefront imaging has been a powerful technology for visual innovations and will guide our innovations even more in the future.
WOMEN IN OPHTHALMOLOGY
BY MARGUERITE MCDONALD, MD, FACS
Twenty-five years ago, there were virtually no female ophthalmologists on the podium. Very rarely were they moderators of sessions at major meetings. There were not many female residents, chief residents nor fellows in any ophthalmic subspecialty. For the female ophthalmologists in private practice and academia, the male vs. female salary differential was substantial. There were vanishingly few female department chairs, if any. Not many female ophthalmologists were on the editorial boards of the peer-reviewed and non-peer-reviewed journals. Lastly, female ophthalmologists of color were few and far between.
Our situation has improved significantly over the last 25 years. Most symposia now have numerous female participants — and often a female moderator. According to the AAO, as of year-end 2020, 40% of ophthalmology residents are women, and 26% of practicing AAO members are women. Females have now established a substantial foothold in the subspecialty fellowship positions as well. The mastheads of our journals reveal that their editorial boards have numerous female members. We are seeing an ever increasing number of residents, fellows and young ophthalmologists who are people of color.
All of this is great news, but there is still some work to be done before the playing field is truly even. According to one study, a salary differential persists between female and male ophthalmologists. Between January and March 2020, Jia et al sent surveys to more than 1,500 practicing ophthalmologists who had recently completed their residency. An examination of the 684 responses showed that female ophthalmologists earned a base salary that was 11%-12% lower per year than their male colleagues. There are still not enough female department chairs, and female ophthalmologists of color could still benefit from more representation in our field.
One example of how far we’ve come occurred when I was a young assistant professor in academia, just a year out of training. Our clinical faculty was voting on whether to fire our best third-year resident, a married woman who had become pregnant with her first child. She had one year left in her training; upon learning of her pregnancy, she immediately started taking extra “on-call” duty to compensate for the time she would be out after her delivery. Her co-residents — all male — were very angry with her. I was the only faculty member who voted against firing her. As the youngest and newest faculty member, I had not said much until this issue presented itself. Her dilemma moved me greatly, and my colleagues listened to my passionate defense, backed down and agreed to let her finish her residency, though she was given only two weeks off after her delivery.
At the last AAO in-person meeting, she approached me. Now a successful ophthalmologist with a large practice, she had tears in her eyes as she thanked me for standing up for her so many years ago. This moment is one that I will treasure forever.
In closing, I want to thank Ophthalmology Management for always being open to great ideas and great work, including those from women! OM, it has been a great pleasure to work with your amazing staff; onward and upward! OM








