Setting your patients up for a successful outcome in refractive cataract surgery requires precise measurements for IOL selection. A growing number of patients select a state-of-the-art extended depth of focus (EDOF) or astigmatism-correcting toric IOL, with the goal of reducing their dependency on glasses following cataract surgery. Patient expectations and satisfaction hinge on the choices of their lens implants and measurements. Up-to-date biometry and imaging techniques are critical tools needed to optimize the success of surgical outcomes and patient satisfaction. In this article, I will review the current options and best practices for the preoperative cataract evaluation.
SS-OCT BIOMETRY
Recent advances in ocular biometry have increased accuracy in refractive outcomes; in particular, swept-source optical coherence tomography (SS-OCT) biometry is a great tool for IOL measurement. SS-OCT currently provides the most accurate measurement of keratometry,1,2 including the measurement of posterior corneal curvature, axial length, anterior chamber depth and white-to-white distance. These newer devices can also image easily through dense cataracts and small pupils and provide access to modern IOL formulas such as the Barrett Universal 2 formula.
CORNEAL TOPOGRAPHY/TOMOGRAPHY
Corneal topography provides a critical, big-picture overview of the cornea that helps the cataract surgeon compare the corneal topography values with biometry data for power, cylinder and axis. Ideally, these cornea measurements should correlate. If the measurements do not correlate, additional imaging or a slit lamp exam for corneal pathology should be performed.
Corneal tomography performed with Pentacam Scheimpflug technology (Oculus) or the Galilei (Ziemer) also includes helpful data for analysis of the posterior corneal curvature and the ability to calculate the power differential for different corneal optical zones. Big picture analysis provided by corneal topography/tomography can help the cataract surgeon identify irregular astigmatism from dry eye or poor tear film quality, anterior basement membrane dystrophy (ABMD), corneal ectasias or prior corneal refractive surgery. Patients with either ABMD or Salzmann’s degeneration may become a candidate for refractive IOL placement if corneal pathology is treated first with a superficial keratectomy. Four to 12 weeks following a corneal smoothing procedure, IOL measurements can usually be done with increased reliability.3
Topography also provides information about the regularity of astigmatism in RK eyes that may benefit from a toric IOL correction.
TREATING OSD
Ocular surface disease (OSD) can and should be optimized to improve candidacy for refractive cataract surgery. For example, treating dry eye with artificial tear use, topical cyclosporine and punctal plugs, along with improving the tear film with warm compresses and other blepharitis treatments, can improve overall measurement quality.
The ASCRS Cornea Clinical Committee recently published a comprehensive algorithm for identifying and managing OSD prior to refractive procedures including cataract surgery.4
MACULA TESTING
Additional preoperative measurements guide appropriate lens choices for patients. In most cases, eyes with macular pathology can be identified with a routine fundus exam. However, macula OCT is a measurement that should be strongly considered as part of the routine refractive cataract surgery evaluation.
Although not currently reimbursed through insurance coverage unless a pathologic finding is identified, OCT imaging of the retina can help identify a subtle epiretinal membrane or lamellar macular hole that may be difficult to see on exam; this is especially useful for dense cataracts. Many refractive cataract surgeons consider a macula OCT as part of the imaging costs of the refractive cataract surgery work-up.
Obtaining macula OCT imaging can reduce the risk of placing an EDOF lens in a patient with macular pathology, which can limit the visual outcome. Identifying this type of pathology at the cataract evaluation can allow for proper counseling and alternative monofocal or toric lens options.
Avoiding measurement surprises
The carpentry proverb “measure twice, cut once” can also be helpful in cataract surgery planning. Repeat or sometimes serial measurements should be considered in certain situations such as dry eye, a history of contact lens wear and in patients with fluctuating vision from prior radial keratotomy surgery.
For instance, Figure 1 shows topography for a patient with a history of 40 years of rigid gas permeable contact lens (RGP) wear demonstrating corneal warpage. A 4-month break from the RGP was needed before the corneal shape stabilized. The patient wore a soft contact lens in the interim, which was adjusted in power as keratometry power steepened from 41.6 D to 42.9 D, and with-the-rule astigmatism increased from 1.9 D to 2.7 D.
Figure 2 shows the final stable topography. The patient elected to undergo surgery using a toric IOL; if IOL calculations had been performed without waiting for the corneal warpage to resolve, an unwanted myopic surprise and under correction of astigmatism would have occurred.
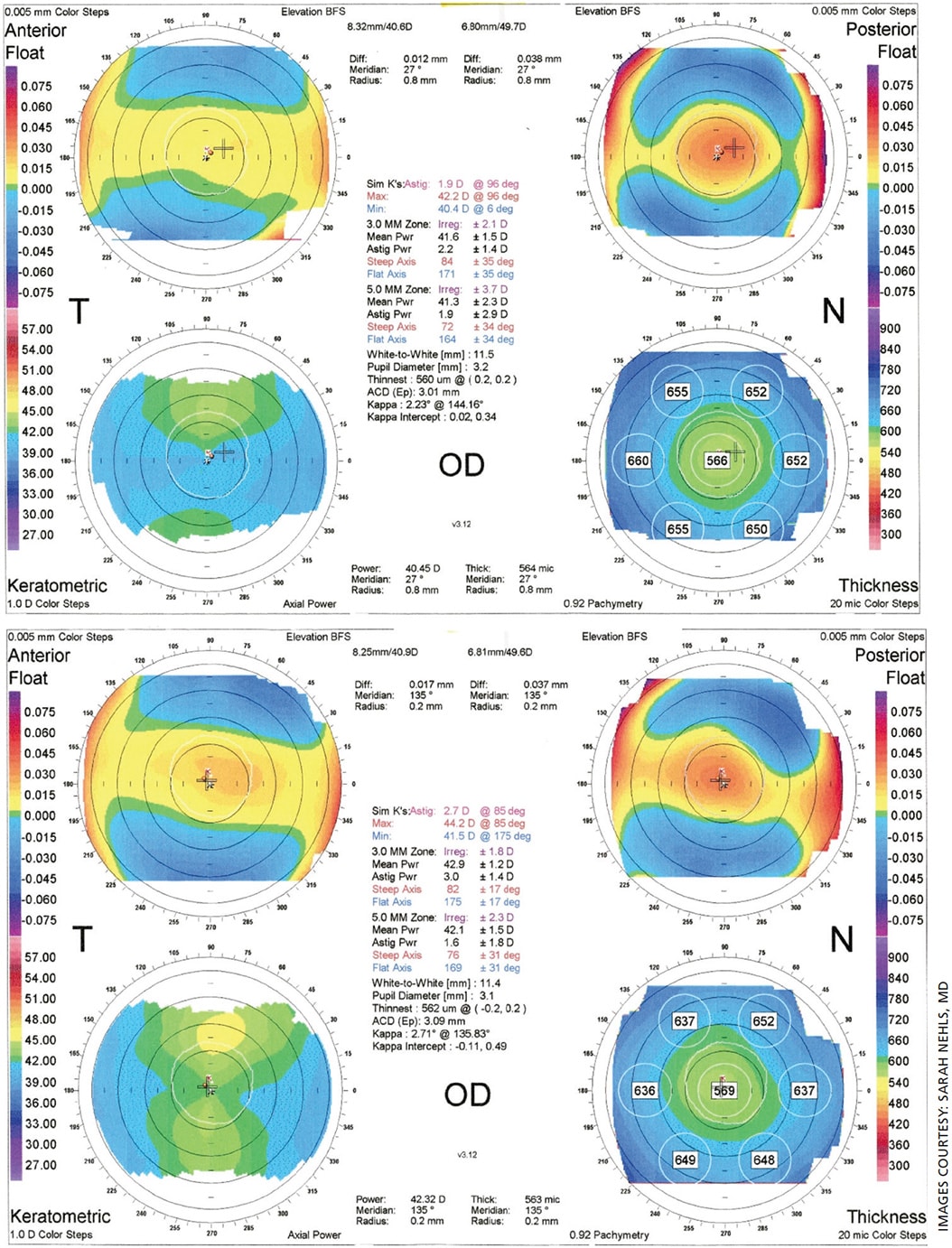
When evaluating eyes with a history of radial keratotomy (RK), topography and biometry should be considered at different times of day to look for fluctuating numbers. Corneal keratometry readings may prove flatter in the early morning compared to later in the day, and using data from the morning may be preferred to avoid a hyperopic surprise.
FUCHS’ DYSTROPHY TESTING
Specular microscopy and ultrasound or optical pachymetry may be indicated in eyes with Fuchs’ endothelial cell dystrophy to assess the risk of inducing pseudophakic bullous keratopathy with cataract surgery. If cornea endothelial cell counts are <500-1000 cells/mm2, a combined cataract and endothelial keratoplasty should be considered. Pachymetry >640 microns is another indication for considering cataract surgery combined with keratoplasty. Clinical history of early morning blur along with slit lamp findings of extensive guttate or stromal fluid clefts also guide assessment for need for a combined procedure. Similarly, I use specular microscopy and pachymetry to evaluate the decompensation risk in an eye with prior penetrating keratoplasty (PKP).5-7
Toric IOL implants can provide significant benefit to eyes with a history of PKP and mostly regular astigmatism as well as in Fuchs’ dystrophy. Based on personal experience, years later, if corneal edema sets in, an endothelial keratoplasty can preserve the benefit of toric lens astigmatism correction.
ULTRASOUND
B-scan ultrasound should be ordered if a mature cataract prevents viewing of the posterior pole. If clear of pathology, refractive cataract surgery could be considered with appropriate patient counseling that a detailed assessment of the macular potential is not possible. Also, A-scan measurement of axial length still plays a role in an eye with a dense cataract if SS-OCT imaging cannot provide a value.
OCULAR DOMINANCE TESTING
Ocular dominance testing is an ancillary test for the refractive cataract surgery patient and is most relevant when monovision correction with cataract surgery is being considered. Ocular dominance can be performed in different ways. I typically have the patient make a circle with their hand and look with both eyes at the 20/200 letter on the Snellen eye chart. Then, I perform an alternate cover test of each eye. The eye in which the image “jumps” is the non-dominant eye, and the eye where the letter stays in alignment is the dominant eye.
In most cases, setting the dominant eye for distance has been considered preferable, although many patients that enjoy monovision also tolerate a cross-dominance outcome (non-dominant eye for distance and dominant eye for reading). In general, if a patient has enjoyed monovision in a contact lens prescription, a monovision surgical plan instead of an EDOF lens is ideal for their cataract surgery, correcting astigmatism as needed with a toric IOL.
CONCLUSION
Measurements for cataract surgery planning are of upmost importance to successful refractive results and patient satisfaction. Core cataract surgery calculation tools include up-to-date biometry and corneal topography/tomography to best manage astigmatism.
Macular OCT should be considered as part of the refractive cataract surgery work up to avoid a surprise from unexpected limited vision outcomes. OSD needs to be optimized proactively to improve the accuracy of surgical measurements. Additional tests such as specular microscopy, pachymetry and ocular dominance testing can be employed to further refine surgical and IOL choices. OM
This work was supported in part by an Unrestricted Grant from Research to Prevent Blindness, Inc. to the University of Wisconsin-Madison.
References
- Yang CM, Lim DH, Kim HJ, Chung TY. Comparison of two swept-source optical coherence tomography biometers and a partial coherence interferometer. PLoS ONE. 2019;14:e0223114.
- An Y, Kang E, Kim H, et al. Accuracy of swept-source optical coherence tomography based biometry for intraocular lens power calculation: a retrospective cross–sectional study. BMC Ophthalmol. 2019;19:30.
- Goerlitz-Jessen MF, Gupta PK, Kim T. Impact of epithelial basement membrane dystrophy and Salzmann nodular degeneration on biometry measurements. J Cataract Refract Surg. 2019;45:1119-1123.
- Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45:669-684.
- Kaup S, Pandev SK. Cataract surgery in patients with Fuchs’ endothelial corneal dystrophy. Community Eye Health. 2019;31:86-87.
- Kohnen T. Compromised corneal endothelium and cataract: how should we decide? J Cataract Refract Surg. 2011;37:1377-1378.
- Seitzman GD, Gottsch JD, Stark WJ. Cataract surgery in patients with Fuchs’ corneal dystrophy: expanding recommendations for cataract surgery without simultaneous keratoplasty. 2005;112:441-446.









