As strides are being made to mitigate the spread of the COVID-19 pandemic, ophthalmic practices are resuming the performance of non-emergent cataract care.
Our multi-site cataract and refractive surgery practice re-opened in early June after being closed for all but emergent cases for approximately 10 weeks. As always, our goal is to provide patients with quality eye care in a safe environment, but now we are doing so with an additional mandate of adherence to CDC guidelines aimed at containing the reverberations of COVID-19. We have implemented enhanced safety protocols to protect patients and staff.
Here, I explain our safety protocols during the pandemic, how cataract surgery was impacted and which protocols may remain standard going forward.
INCREMENTAL REOPENING
Ophthalmic Consultants of Long Island (OCLI) Vision is a tertiary referral center. Patients and referring doctors know that if there’s a problem, it can be managed by someone in our practice. We have specialists in every aspect of ophthalmology, and because of our size we have resources that other practices might not. This allowed us to continue functioning despite the unstable environment we’ve had since spring. We maintained a presence of ophthalmologists seeing patients on an emergent basis that kept the practice mildly busy and fulfilled our obligation to care for patients who needed to be seen.
The plan is to continue providing quality eye care while maintaining patient safety and keeping the busiest offices open and running smoothly. We have 30 offices in New York, Connecticut, New Jersey and Pennsylvania, and we are reopening incrementally starting with our largest and busiest sites. Focusing on the busiest offices, with the greatest demand, we reached about 90% capacity in August. Some patients are still at the point where they would rather wait a few more months until things are a bit more stable, so we have continued to give patients the option to schedule telemedicine visits via phone or video chat. Also, staffing is limited for the time being because some personnel have not returned.
SAFETY AND SOCIAL DISTANCING
We are doing everything possible to maintain social distancing and limit the number of people our patients encounter during their appointment.
Our schedules have been adjusted in an attempt to enforce social distancing, with the primary changes being less throughput and the addition of steps to sterilize the equipment and exam rooms between patients. Initially, we were seeing about half the number of patients that we would normally see in an hour. As staff have become accustomed to our new routines, we have dramatically increased patient flow.
Strict measures are employed to minimize unnecessary interaction for the benefit of patients and staff (Figure 1), including:

- We reconfigured waiting room seating, with only six seats placed at a safe distance apart.
- We removed superfluous items for the waiting room, such as magazines and a self-serve water station.
- We required all patients to wear a mask, and their temperature is taken upon arrival.
- We have a sign-in sheet that asks about recent travel or exposure to COVID-19, and patients with possible exposures or history of recent travel are invited to return at a later date.
- All doctors and staff wear masks at all times.
- A plexiglass panel separates patients and front desk personnel, and a large breath shield provides protection to both patients and doctors during slit lamp exams (Figure 2).
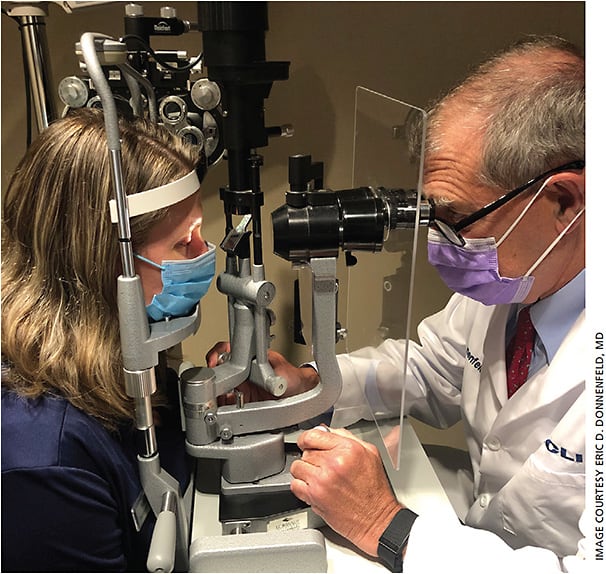
Figure 2. Plexiglass between patients and doctors during exams is among the numerous protocols implemented at OCLI Vision to mitigate COVID-19 virus transmission. - We asked patients to wait outside or in their vehicle until it is their turn to be seen. Only children and elderly persons who need assistance are allowed to be accompanied by a family member.
THE NEW NORMAL
As we implement these changes and scrutinize our behavior, everything is being reassessed with an eye to the future, and we are considering what will stay and what will go. For instance, patients now spend a lot less time in the office, and they like being seen by the doctor and getting in and out in a reasonable time. Going forward, we will try to keep the waiting rooms empty and expedite the work-up and the examination.
Interestingly, we’ve seen heightened interest in premium IOLs during this time. Several scenarios may account for this. For example, patients wearing masks are uncomfortable with persistent fogging of their glasses. In addition, patients may have a sense of insecurity in the current environment, and improving their quality of vision can be a simple way to exert influence during a time when they are feeling otherwise powerless. Furthermore, patients whose employment has not been affected by the pandemic may have an excess of disposable income because their travel and entertainment budgets are going untouched. From that perspective, it’s a perfect time to opt for a refractive IOL. The interest in premium pathways has been a bit of a surprise to me, and I’m hoping this is something that remains as we navigate the new normal.
SAFER SURGERY
Surgical initiatives that we have further committed to in response to COVID-19 include intraoperative drug delivery systems such as Dexycu (dexamethasone intraocular suspension 9%, EyePoint Pharmaceuticals), Dextenza (dexamethasone ophthalmic insert 0.4 mg, Ocular Therapeutix) and Omidria (phenylephrine 1% and ketorolac 0.3% intraocular solution). All of these options enable the reduction or elimination of postoperative eyedrops that may require a family member or caregiver to come in contact with the patient.
Typically, patients who are predisposed to substantial postoperative inflammation, such as those who have a history of uveitis, diabetes, epiretinal membrane or prior retinal surgery, are excellent candidates for sustained-release steroid formulations such as Dexycu or Dextenza. As we deal with the additional safety concerns associated with COVID-19, these intraoperative delivery systems are a helpful adjunct in all cataract surgery cases. Dexycu is a cohesive, bioerodible liquid steroid depot that is injected into the ciliary sulcus at the end of cataract surgery, where it delivers a tapering dose of dexamethasone for about 21 days.1,2 Dextenza, another dexamethasone delivery system, uses a resorbable hydrogel device inserted at the end of surgery into the canaliculus of the lower lid where it remains in place for about 30 days, delivering a tapered dose of medication to the ocular surface.3 Omidria is used during cataract surgery to prevent miosis and reduce postoperative pain. Also, it obtains high vitreous levels of the nonsteroidal anti-inflammatory drug ketorolac and has been shown to reduce the incidence of cystoid macular edema.4,5 Combining Omidria with a sustained-release steroid formulation allows patients to avoid postoperative topical anti-inflammatories.
Whenever patients are reliant upon fewer postoperative eyedrops, it’s beneficial for them and their family members or caregivers. When you factor in the additional concerns associated with COVID-19, reducing or eliminating postoperative drops is a gamechanger. Not having to instill postoperative drops means a family member doesn’t have to drive to the pharmacy, maintain social distancing, don a mask to purchase the prescription and travel to the patient’s home several times a day to help with the drops. What’s more, because patient adherence is not an issue when sustained-release drug delivery systems are used, I’m very comfortable with fewer follow-up visits, for which patients and their family members are grateful.
Therefore, we have found combined minimally invasive glaucoma surgery (MIGS) and cataract surgery to be especially helpful under these challenging circumstances. We’ve been performing MIGS on every cataract patient who has glaucoma, but now there’s a tendency to go even further, employing additional MIGS adjuncts that were less commonly used before. For instance, glaucoma specialists in our practice have been doing a lot more selective laser trabeculoplasty, and excimer laser trabeculectomy is another exciting treatment that has gained some traction in our practice these days.
WHAT’S AHEAD
As far as the COVID-19 pandemic goes, we’re not out of the woods. Some geographic areas are doing exceedingly well at minimizing new cases and deaths, but we’re also seeing spikes in the numbers of cases and casualties in other regions of the country. This suggests that the virus has a high probability of remaining a threat if we are not resolute in maintaining social distancing, wearing masks, eliminating indoor gatherings and doing everything possible to practice consistent hygiene and minimize interaction. This is especially important for elderly and high-risk patients — many of whom populate our cataract surgery dockets. Given these circumstances, we need to continue exploring new ways to make the postoperative period easier and safer for patients to navigate, and to fully embrace those that we’ve already found. OM
REFERENCES
- Donnenfeld E, Holland E. Dexamethasone intracameral drug-delivery suspension for inflammation associated with cataract surgery: a randomized, placebo-controlled, phase III trial. Ophthalmology. 2018;125:799-806.
- Donnenfeld ED, Solomon KD, Matossian C. Safety of IBI-10090 for inflammation associated with cataract surgery: Phase 3 multicenter study. J Cataract Refract Surg. 2018;44:1236-1246.
- Tyson SL, Bafna S, Gira JP, et al. Multicenter randomized phase 3 study of a sustained-release intracanalicular dexamethasone insert for treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2019;45:204-212.
- Rosenberg ED, Nattis AS, Alevi D, et al. Visual outcomes, efficacy, and surgical complications associated with intracameral phenylephrine 1.0%/ketorolac 0.3% administered during cataract surgery. Clin Ophthalmol. 2017;12:21-28.
- Visco DM, Bedi R. Effect of intracameral phenylephrine/ketorolac 1.0%/0.3% on postoperative cystoid macular edema, iritis, pain, and photophobia following cataract surgery [published online ahead of print, 2020 Mar 26]. J Cataract Refract Surg. 2020.









