Dry eye disease (DED) is an extremely common pre-existing and underdiagnosed condition in patients presenting for cataract surgery, due to their advanced age.1,2
Why
There are three major reasons we should screen and aggressively treat DED before refractive cataract surgery:
- The tear film is the most important refracting surface of the eye. An unstable or unhealthy tear film can result in inaccurate biometry and keratometry readings and, thus, suboptimal refractive outcomes postoperatively.3-5 Precise keratometry relies on a good reflection of the mires from the corneal surface, which can be compromised in an unstable tear film. This is particularly important for DED patients interested in presbyopia-correcting IOLs: They may not achieve an accurate refractive outcome with these IOLs.
- Cataract surgery is likely to either induce or exacerbate preexisting DED in a significant portion of patients. DED complaints, such as gritty sensation, eye fatigue, vision fluctuation, and general ocular discomfort, are common complaints in more than 50% of patients after cataract surgery.2 Also noteworthy: The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study shows the majority of patients screened for cataract surgery did not complain of DED symptoms, yet up to 80% scheduled for cataract surgery had evidence of DED, with 77% having corneal staining, and 63% having a TBUT < 5 seconds. Furthermore, only a small percentage of these patients (22%) had a previous DED diagnosis.1 While usually transient, DED is one of the most common reasons for patient dissatisfaction or unhappiness with their results postoperatively. These patients should be educated that symptoms are likely to become more noticeable postoperatively, even if they are asymptomatic preoperatively.
- Treating lid disease and blepharitis and eliminating bacterial overgrowth helps to reduce the risk of postoperative infection or endophthalmitis.6-11 Meibomian gland dysfunction (MGD) is thought to be the leading cause of DED, with more than 85% of these patients having MGD.12
The American Academy of Ophthalmology advocates that all patients undergoing cataract surgery be evaluated and treated for DED preoperatively.13
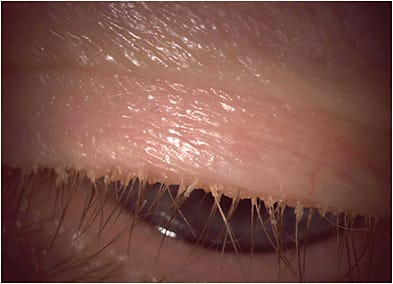
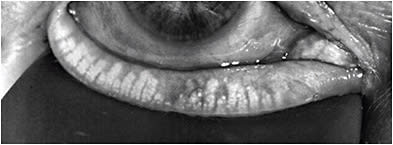
How to Diagnose
DED is a multifactorial disease that can be complex to diagnose and treat. Nevertheless, an organized approach can facilitate the diagnosis, treatment recommendations, and management plan.
A first step to screening is the use of a patient-reported symptom questionnaire, such as the Standardized Patient Evaluation of Eye Dryness (referred to as SPEED) questionnaire or the Ocular Surface Disease Index (referred to as OSDI), to evaluate the patient’s perception of issues with their ocular surface. Symptomatic patients can trigger point-of-care diagnostic tests, such as tear osmolarity, MMP-9, and meibography.
Topography can be used for evaluating OSD. We want to determine whether patients’ visual complaints are consistent with the degree of the cataract and not due to other comorbidities. Not only can topography aid in detecting signs of DED, but other pathologies, such as epithelial basement membrane dystrophy, irregular astigmatism, and keratoconus—that may not otherwise be seen on exam at the slit lamp—can be detected.
Tear break-up time, evaluating the lids and lid margins, quality of tear film, and corneal/conjunctival staining can help distinguish between aqueous deficient and evaporative DED, and, thus, should be incorporated in every patient exam. A reduction in the blink frequency and incomplete blinks have been associated with excessive digital device use and subsequent DED. Measurements of these blinking parameters, using an interferometer, is, therefore, useful in assessing for DED. Tear volume is occasionally measured while assessing DED and may assist in distinguishing between aqueous deficient and evaporative DED. Several methods, including the Schirmer’s test, phenol red thread, strip meniscometry, and noninvasive methods can be used for tear volume.
Using a combination of these diagnostic tools, along with a good history and careful clinical exam—paramount—can usually provide us with whether DED is present, how significant it is and how severe it is.
How to Treat
Several algorithms have evolved to help simplify DED management for clinicians. They should not be seen as a rigid stepwise approach, but rather as an organizational tool to aid treatment decisions. Some of the most recent algorithms include: the Tear Film & Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II) algorithm (https://bit.ly/2FzyfN8 ); the Cornea, External Disease, and Refractive Society (CEDARS) algorithm (https://bit.ly/34iWIk4 ), and the ASCRS Preoperative OSD Algorithm (see “Research Spotlight,” p.40.)
The goal of treating DED prior to cataract surgery is to reduce inflammation and restore ocular surface homeostasis as quickly and efficiently as possible. I have found that educating the patient about the importance of optimizing the health of the ocular surface helps to improve compliance.
An omega-3 fish oil is included in the mentioned DED algorithms.14-18 Warm compresses for lid hygiene and preservative-free tears to keep the ocular surface moist are also recommended.
Patients who have aqueous deficient or evaporative DED can be prescribed an immunomodulator, such as cyclosporine 0.09% (Cequa, Sun Pharmaceutical Industries, Inc.), cyclosporine 0.05%, (Restasis, Allergan) or lifitegrast 5% (Xiidra, Novartis) before cataract surgery. My colleagues, John A. Hovanesian, MD, Eric D. Donnenfeld, MD, Jack Holladay, MD, and I performed a study to determine whether proactive treatment of the ocular surface (even in relatively asymptomatic patients) could improve refractive prediction in cataract surgery. Our findings: Biometry after lifitegrast treatment predicted outcomes more accurately than prior to treatment.4 A similar study on cyclosporine is also underway.
Using a short course of a topical steroid in conjunction with an immunomodulator can rapidly reduce inflammation on the ocular surface.
Punctal plugs are also an option once the inflammation is addressed. These treatments must be balanced with the reduction of the BAK burden from topical drops, including glaucoma medications and artificial tears.
Patients who have evidence of MGD should consider undergoing an in-office treatment. LipiFlow Thermal Pulsation System (Johnson & Johnson Vision), iLux (Alcon), TearCare (Sight Sciences) or intense pulse light therapy fall under this category.
Cynthia Matossian, MD, presented data at last year’s ASCRS meeting that shows thermal pulsation treatment prior to cataract surgery improves the accuracy of keratometry and subsequent surgical decision-making.3 After addressing the gland obstruction, traditional treatments, such as lid hygiene and warm compresses, seem more effective in helping to maintain the benefit of the thermal pulsation therapy. More advanced cases of DED may benefit from autologous serum tears, an amniotic membrane and/or prosthetic replacement of the ocular surface ecosystem (referred to as PROSE).
Patients are asked to return for repeat biometry 4 to 6 weeks after DED treatment is initiated. If the ocular surface is still not optimized, we shouldn’t hesitate to delay surgery until the surface is healthy enough to generate accurate measurements.
If optimizing the ocular surface or obtaining reproducible keratometry and topography is not possible, I am hesitant to recommend a presbyopia-correcting IOL. I have found that most patients compliant with DED treatment experience less refractive surprises.
Improving Outcomes
Screening for DED and optimizing the health of the ocular surface is critical to maximizing patient satisfaction and outcomes after cataract refractive surgery. CP
References
- Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG, Goldberg DF. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423-1430.
- Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. 2018;44(9):1090-1096.
- Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672-1677.
- Hovanesian J, Epitropoulos A, Donnenfeld ED, Holladay JT. The Effect of Lifitegrast on Refractive Accuracy and Symptoms in Dry Eye Patients Undergoing Cataract Surgery. Clin Ophthalmol. 2020 Sep 16;14:2709-2716. doi: 10.2147/OPTH.S264520. PMID: 32982163; PMCID: PMC7502384.
- Matossian C. Effect of thermal pulsation system treatment on keratometry measurements prior to cataract surgery. Paper presented at: Annual meeting of the American Society of Cataract and Refractive Surgery; San Diego, CA; May 6, 2019.
- Speaker MG, Milch FA, Shah MK, Eisner W, Kreiswirth BN. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98(5):639-649.
- O’Brien TP, Arshinoff SA, Mah FS. Perspectives on antibiotics for postoperative endophthalmitis prophylaxis: potential role of moxifloxacin. J Cataract Refract Surg. 2007;33(10):1790-1800.
- Lindstrom RL. The effects of blepharitis on ocular surgery. Ocul Surf. 2009;7(2 Suppl):S19-S20.
- Galor A, Goldhardt R, Wellik SR, Gregori NZ, Flynn HW. Management strategies to reduce risk of postoperative infections. Curr Ophthalmol Rep 2013;1(4):161-168.
- Stead R, Stuart A, Keller J, Subramaniam S. Reducing the rate of cataract surgery cancellation due to blepharitis. Eye (Lond). 2010;24(4):742.
- Niyadurupola N, Astbury N. Endophthalmitis: controlling infection before and after cataract surgery. Community Eye Health. 2008;21(65):9-10.
- Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478.
- American Academy of Ophthalmology Cornea/External Disease Committee. Dry Eye Syndrome. San Francisco, CA: American Academy of Ophthalmology; 2018. Preferred Practice Pattern Guidelines. Available at: https://www.aao.org/preferred-practice-pattern/dry-eye-syndrome-ppp-2018 .
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-574.
- Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: Dry eye disease and associated tear film disorders – new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27(Suppl 1):3-47.
- Starr CE, Gupta PK, Farid M et al; ASCRS Cornea Clinical Committee. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669-684.
- Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35(9):1185-1191.
- Giannaccare G, Pellegrini M, Sebastiani S, et al. Efficacy of omega-3 fatty acid supplementation for treatment of dry eye disease: a met-analysis of randomized clinical trials. Cornea. 2019;38(5):565-573.









