If there ever was a tipping point for at-home patient monitoring and treatment, 2020 was it. As physicians and patients learn to live with COVID-19, a variety of technologies are becoming more critical for keeping patients safe and healthy. Once a matter of convenience and compliance, tools that enable at-home care have become a more critical need. The earlier a diagnosis is made, the earlier treatment can start, which means a better chance at limiting or slowing vision loss. It is important that patients are reminded, however, that home monitoring does not mean they can skip regular in-office visits altogether.
In April 2020, the FDA published new guidance designed to “expand the capability of remote ophthalmic assessment and monitoring devices[,] to facilitate patient care while reducing patient and healthcare provider contact and exposure to COVID-19.”1 The guidance asserts that for the duration of the COVID-19 emergency, the FDA does not intend to object to limited modifications to the indications, functionality, hardware and/or software of many ophthalmic devices designed for at-home use, as long as they don’t present “undue risk.”1
Here is a sampling of the latest at-home technologies patients are using today, along with some on the horizon.
MONITORING
Alleye
The Alleye app (Oculocare) is a smartphone- and tablet-compatible app designed to enable early detection of AMD and diabetic macular edema, as well as at-home monitoring of an existing retinal disease’s progression. To perform the test, a patient places 12 points along an invisible line connecting two outer points. The patient performs the test one eye at a time. Alleye is developed by ophthalmologists and received FDA clearance in 2018.2,3
Patients need a recommendation by an eye-care professional to access Alleye. An annual license is required to connect the Alleye app to an eye-care professional’s account. This test is appropriate for patients diagnosed with AMD who want to be sure to schedule a doctor’s visit at the right time.
“Alleye is among the few health apps available from app stores that underwent rigorous clinical evaluation,” says Lucas Bachmann, CEO and cofounder of Oculocare, adding that its “development was based on a principled approach where both testing performance and usability were the focus.”
Amsler grid
Amsler grid testing is an effective way to monitor macular function and determine a patient’s visual field.4 Patients can access an electronic Amsler grid test from their computer, tablet or mobile device, making at-home testing easy. The Macular Degeneration Foundation has made the Amsler grid available on its website as both an image file and a phone app in addition to several versions of the test available online.
ForeseeHOME
Notal Vision’s ForeseeHOME is a commercially available, at-home device designed to facilitate the early detection of wet AMD. Patients that use the test spend 6 minutes per day (3 minutes per eye) clicking a target point on a digital image. Reports are sent to Notal Vision daily, and clinicians receive monthly reports on patient progress. According to Notal Vision, patients using ForeseeHOME had a 94% rate of preserving driving vision (20/40 or better) at wet AMD diagnosis compared to 62% with standard methods of detection alone.5

The company has established a cloud-based infrastructure that connects health-care providers. ForeseeHOME is the first application of Notal Vision’s cloud-based platform; it uses a patented technology called Preferential Hyperacuity Perimetry (PHP) to detect visual distortions, often before the patient notices any visual symptoms.
The device was tested in a large, prospective randomized clinical trial called the HOME Study, on patients who were also in the AREDS II study, notes Nancy Holekamp, MD, who served on the Data and Safety Monitoring Committee. “We terminated the study early because the ForeseeHOME was so much more effective in detecting the conversion from dry to wet AMD compared to the standard of care (Amsler grid) that it became unethical not to offer the ForeseeHOME device to all patients with bilateral drusen,” she says.
Dr. Holekamp recommends the ForeseeHOME device in her practice at Pepose Vision Institute, St. Louis, for patients “who want the latest technology for early detection of wet AMD.
Home OCT
New advances in optical coherence tomography (OCT) technology promise to move routine examinations from the practice to a patient’s home. Notal Vision’s Home OCT is a light-weight device designed for technician-free operation. It is intended to complement a practitioner’s current disease monitoring strategies by providing additional clinical data points. The Home OCT received FDA Breakthrough Device designation in late 2018 and is pending regulatory approval.6
In June, Notal Vision announced an ongoing, longitudinal usability trial demonstrating “the daily dynamics of retinal fluid in patients receiving anti-VEGF treatment for wet AMD.”7 According to the company, current trial results indicate that 90% of AMD patients can self-operate and generate analyzable images following a 2-minute tutorial, with formal data pending publication.
“Home OCT will provide physicians with inter-visit disease knowledge and help us reduce the treatment and monitoring burden on patients and their caregivers,” said Anat Loewenstein, MD, the study’s principal investigator and the director of the department of ophthalmology at Tel Aviv Medical Center, in a news release. “Daily monitoring of disease activity will aid retina specialists in achieving the desired visual acuity outcomes for their patients.”
Home Vision Monitor app
Home Vision Monitor (HVM) (Roche/Genentech) is an FDA-cleared 510(k) Class 1 medical device designed to detect early vision changes in patients with AMD and diabetic eye disease. As an app, patients can use HVM on their smartphone or tablet and typically test their vision one to two times a week using a simple shape discrimination hyperacuity test to detect vision changes. It is currently being piloted at Moorfields Eye Hospital in the United Kingdom.
Patients with active neovascular AMD (nAMD) require regular eye injections with anti-vascular endothelial growth factor therapy, but the need for repeat injections varies widely among individuals. The app can be used to personalize care and minimize time spent in the clinic and reception rooms.
In the absence of regular clinic visits, the HVM app can also provide a safety net for patients with stable nAMD, who generally require monitoring every 6 weeks to 3 months. If a significant change in the vision result is detected from one session to the next, this triggers an alert to the prescribing physician who can then decide whether the patient needs to come into the clinic for an assessment.

Icare HOME Tonometer
Icare USA’s Icare HOME Tonometer addresses ophthalmologists’ concern that their IOP check on their glaucoma patients during office visits only gives them a one-minute snapshot of a patient’s true IOP during the day — while the IOP during the other 99% of the patient’s day remains a mystery. The Icare HOME Tonometer enables clinicians to obtain a true idea of the patient’s IOP throughout the day and night, the company says, giving them a better understanding of their patient’s disease state and the success of their IOP-lowering interventions.
The tonometer is a hand-held, anesthetic drop-free instrument — Icare’s tagline for the product is, “No anesthesia, no air puff.” A small probe touches the cornea and evaluates the impact duration, deceleration and other motion parameters. It then calculates IOP based on the deceleration and rebound time. The HOME tonometer records the IOP measurements as well as additional data, which is not displayed to the patient.
The clinician later downloads the measurement data via Icare LINK software.
TREATMENT DEVICES
NuLids
NuLids is an all-natural home treatment that provides symptomatic relief for patients suffering from dry eye, blepharitis or Demodex. The NuLids device stimulates the meibomian glands via external application to the upper and lower eyelids. Patients use the device for one minute each day.
A clinical study for the device showed a statistically significant improvement in signs and symptoms of DED, as demonstrated by improved OSDI, tear osmolarity, tear breakup time, meibomian gland score, meibomian gland yielding liquid secretions and ocular staining score.8 During the clinical study, patients reported no adverse events, corneal trauma or conjunctival trauma, and 91% of participants agreed or strongly agreed that the device was easy and convenient to use.
According to President Robert Foster, there have been more than 750,000 at-home patient treatments to date. In a survey of more than 200 users, 84% said NuLids is very effective at reducing symptoms, 94% reported it convenient, comfortable and easy to use, 91% said it was a good, very good or excellent value and 71% had reduced their use of artificial tears, lubricants, gels and/or discontinued use of prescription drops. Further, the device is associated with a 95% retention rate of patients for the eye-care provider who dispensed the device, according to the company.
“The NuLids system is a great tool for patients to maintain their eyelid hygiene at home and take control of their health,” says Wendy Gross, OD, Gordon Schanzlin New Vision Institute, La Jolla, Calif.
iTear100
Olympic Ophthalmics’ iTear100 device is a prescription neurostimulation technology that temporarily increase acute tear production. It received FDA approval in May. The handheld and battery-powered device is applied externally to the anterior ethmoidal nerve.
TEAR 2 (CLP OO7) was a randomized, controlled study of 59 subjects, and TEAR 1 (CLPOO2) was a single-arm study with a primary endpoint at 30 days and enrolled 108 subjects.9 In TEAR2, the device demonstrated a 22-mm change in Schirmer score vs sham with no device-related adverse events.
TEAR 1 demonstrated the continued ability to increase immediate tear production to 30 days. At that time point, a 9.4-mm change from unstimulated to stimulated tear production was observed. The device was well-tolerated, with a 2% to 3% incidence of dizziness and headache during the study. The production of tears and patient satisfaction was seen in even the most severe patients, according to Laura M. Periman, MD, who participated in the Phase 3 FDA trials for the iTear 100.
“Neurosensory compromise is part of the TFOS DEWS II definition of dry eye disease,” says Dr. Periman, director of Dry Eye Services and Clinical Research, Periman Eye Institute, Seattle, Wash. “Clinically, addressing the neurosensory compromise is an important component of advanced dry eye treatment and patient care. The iTear100 is an exciting approach to the neurosensory compromise. We offer it to advanced dry eye patients and patients where there may be neurosensory compromise, including dry eye after cataract surgery or after refractive surgery and to autoimmune patients.”
COMMUNICATION, COMPLIANCE
EyeDropAlarm
EyeDropAlarm (Kayur Shah) is a free app that helps patients remember to take their eyedrops. Designed for patients with a range of ocular conditions — including glaucoma, dry eye, ocular allergies and uveitis — the app is user friendly and does not require an account login to use. The app includes built-in algorithms for tapering and staggering drops, and drop frequency can be set to one to 24 times per day.
“I find it to be a useful tool to help patients remember to take their drops on time, especially if they have multiple medications,” says Lisa Nijm, MD, JD, founder and medical director at Warrenville EyeCare and LASIK in Illinois.
Glaucoma App
The Glaucoma App is a free app created by Wills Eye Glaucoma Research Center and Drexel University. The app was created to improve patient compliance and disease state knowledge. It offers eyedrop reminders, appointment reminders, instructional videos, an IOP tracker, medical and ocular data storage and visual field tutorials.
MDbackline
MDbackline is a secure, cloud-based web service that automates conversations with eye-care patients before and after office visits to collect information on patients’ lifestyle and visual needs, as well as to deliver educational materials customized to the doctor’s offerings. This serves to improve care for patients.
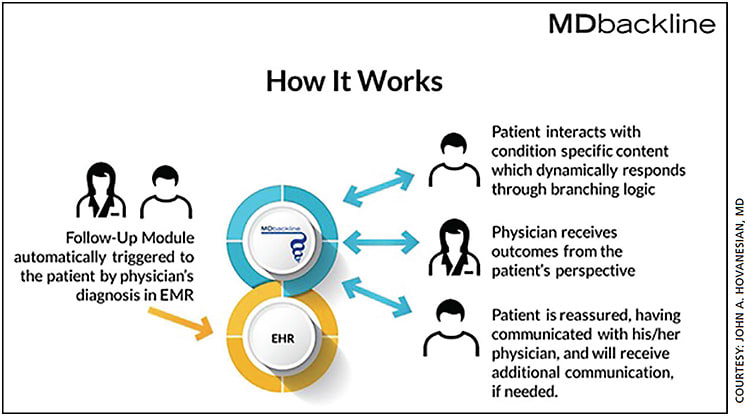
Founder John A. Hovanesian, MD, in clinical practice in Laguna Hills, Calif., notes three areas where ongoing coaching, reminding and checking in with patients are helpful: glaucoma (where compliance is the big issue), dry eye disease and cataract surgery. MDbackline anticipates the needs of patients and automatically contacts them by text and email to offer real-time communication, whether it be to remind patients to take their glaucoma drops or to provide education regarding IOL choices for cataract surgery.
“With glaucoma for example, MDbackline provides a way to track patients compliance and find out if they are experiencing side effects with their drops,” says Dr. Hovanesian. “Unless we ask patients questions about problems they may be having, we can miss important information that can help them stay on track with their treatment.”
The system is configurable to the physician’s needs, and all information exchanged online is secure and protected. Patients feel taken care of as they get real-time information direct from the doctor’s office, Dr Hovanesian adds. Based on patients responses, communication can be escalated as needed. OM
REFERENCES
- U.S. Fooda and Drug Administration. Enforcement Policy for Remote Ophthalmic Assessment and Monitoring Devices During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency. Accessed Sept. 15, 2020. https://tinyurl.com/y2jsfrxd
- PR Newswire. Oculocare’s Alleye Receives FDA 510(k) Clearance for Monitoring Eyesight in AMD. Accessed Sept. 15, 2020 https://tinyurl.com/y5rv7sgm .
- Schmid MK, Faes L, Bachmann LM, Thiel MA. Accuracy of a self-monitoring test for identification and monitoring of age-related macular degeneration: a diagnostic case-control study. Open Ophthalmol J. 2018;12:19-28.
- Tripathy K, Salini B. Amsler Grid. Treasure Island (FL): StatPearls Publishing. 2020.
- Chew EY, Clemons TE, Bressler SB, et al; AREDS2-HOME Study Research Group. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121:535-544.
- Notal Vision. Clinical trial data on file. Accessed Sept. 15, 2020. https://notalvision.com/technology/home-oct .
- GlobeNewswire. Home OCT longitudinal home-based study with patient self-operated device has begun. Accessed Sept. 15, 2020. https://tinyurl.com/y56co8qn .
- Schanzlin D, Olkowski J, Coble J, et al. Efficacy of self-administration of a personal mechanical eyelid device for the treatment of dry eye disease, blepharitis and meibomian gland disease. J Dry Eye Ocul Sur Dis. 2020;3:e1-e5. doi.org/10.22374/jded.v3i1.25
- PR Newswire. Olympic Ophthalmics Presents Clinical Evidence for iTEAR100. Accessed Sept. 15, 2020. https://tinyurl.com/y2fsyuo4








