Inflammation after cataract surgery is more common than once thought. We see a high incidence of rebound or persistence inflammation, especially in certain populations. Although often self-limited, inflammation can lead to increased complications, such as posterior capsule opacification, posterior synechiae, corneal edema and cystoid macular edema.1
Traditionally, topical drops were the mainstay of treatment for prevention and treatment of inflammation. New technology in pharmacological treatment of ocular inflammation has expanded options for patients in the perioperative period for cataract surgery and lens- and cornea-based refractive surgery.
“THE OLD”: TOPICAL DROPS
NSAIDs
A multitude of anti-inflammatory treatments are now available to aid in achieving rapid and complete resolution of postoperative inflammation. Steroid and nonsteroidal anti-inflammatory (NSAID) drops are the traditional agents prescribed, but there is no universally accepted regimen. The classic weekly 4-3-2-1 taper schedule of a steroid and NSAID drop is still often prescribed, and, when combined with an antibiotic drop, patients are left with a month-long schedule of well over 100 doses of drops to instill. Not only is this quantity and frequency burdensome for patients and caregivers, but these drops are often toxic to the ocular surface.
Tailoring and customizing the postoperative drop regimen, especially in patients with preexisting ocular surface disease, can speed visual recovery and improve the surgical journey. Minimizing preservatives, duration of treatment and frequency of dosing may lessen surface dryness that can increase after cataract surgery while still adequately treating postoperative discomfort and inflammation.
NSAID or not?
While no randomized controlled trial has shown definitive benefit of an NSAID drop following cataract surgery, several meta-analyses and case series suggest benefit of postoperative NSAIDs, especially in diabetics.2,3 The AAO reported in 2015 that, while there is a lack of level I evidence to support the long-term benefit of NSAID therapy following cataract surgery, dosing of NSAIDs before surgery may speed visual recovery in the first several weeks postoperatively.4
Newer NSAIDS are designed for less frequent dosing (Table 1), which improves compliance and potentially reduces cost for the patient — less frequently dosed drops typically last longer, and one bottle could potentially last for both eyes. Many of the newer NSAID drops have twice- or even once-daily dosing while containing half the concentration of preservative compared with the traditionally prescribed ketorolac, thus minimizing toxicity to the ocular surface while still offering the benefit of reduced discomfort and inflammation. Caution should still be taken, however, as NSAIDs as a class can still decrease corneal sensation and slow or delay corneal healing in at-risk patients.
| NSAID | Manufacturer | Dosing | Duration | Bottle size | Preservative |
| Acular (ketorolac tromethamine (0.5%) | Allergan | Four times daily | 2 weeks | 10 mL | Benzalkonium chloride 0.01% |
| Prolensa (bromfenac 0.07%) | Bausch + Lomb | Once daily | 2 weeks | 3 mL | Benzalkonium chloride 0.005% |
| Ilevro (nepafenac 0.3%) | Novartis | Once daily | 2 weeks | 4 mL | Benzalkonium chloride 0.005% |
| BromSite (bromfenac 0.075%) | Sun Ophthalmics | Twice daily | 2 weeks | 5 mL | Benzalkonium chloride 0.005% |
Reduced dosing options
While steroid drops are a mainstay of treatment for many surgeons in treating post-operative inflammation, newer steroid drop options simplify the traditional course by allowing for reduced inflammation with less frequent dosing. Difluprednate has been shown to successfully reduce postoperative inflammation and pain with twice-daily dosing for 2 weeks, followed by a 2-week taper.5 Submicron loteprednol etabonate ophthalmic gel 0.38% (Lotemax SM, Bausch + Lomb) appears effective in twice-per-day administration for 14 days, and loteprednol etabonate 1% (Inveltys, Kala Pharmaceuticals) is effective in controlling pain and inflammation alone, without an NSAID drop, at twice-daily dosing for 14 days following cataract surgery.6,7 These dosing regimens offer significantly less frequent drop instillation for shorter duration than the traditional taper, greatly simplifying patient discussions and execution.
ImprimisRx offers many unique compounded combination drops that also greatly simplify the post-operative drop schedule. Several antibiotic-steroid, antibiotic-NSAID-steroid and steroid-NSAID options are available that provide uniform dosing of multiple agents, administered from a single bottle. (Table 2 lists steroid drop options.)
| Steroid | Manufacturer | Bottle size | Preservative |
| Pred Forte (prednisolone acetate 1%) | Allergan | 5, 10 mL | Benzalkonium chloride 0.006% |
| Flarex (fluorometholone acetate 0.1%) | Eyevance | 5, 8 mL | Benzalkonium chloride 0.01% |
| Durezol (difluprednate 0.05%) | Novartis | 5, 8 mL | Sorbic acid 0.1% |
| Lotemax SM (loteprednol etabonate 0.38%) | Bausch + Lomb | 10 mL | Benzalkonium chloride 0.003% |
| Lotemax (loteprednol etabonate 0.5%) | Bausch + Lomb | 5, 10, 15 mL | Benzalkonium chloride 0.01% |
| Inveltys (loteprednol etabonate 1%) | Kala Pharmaceuticals | 10 mL | Benzalkonium chloride 0.01% |
INTRAOPERATIVE OPTIONS
Tri-Moxi
Many patients expect a simple postoperative regimen. Unfortunately, they struggle with eyedrop instillation and understandably find several-week, multi-drop regimens burdensome. Fortunately there are new delivery methods that can remove the burden of drop compliance from the patient.
Tri-moxi (ImprimisRx) is a compounded intravitreal injection of triamcinolone acetonide-moxifloxacin that is administered in a transzonular approach following cataract surgery (Figure 1). This agent is sometimes combined with a postoperative NSAID drop. While retrospective reviews have shown this agent to be a promising method to control intraocular inflammation after cataract surgery, there is concern for an increased risk of retinal tears, detachments or zonular trauma with the transzonular method of delivery.8

Dextenza
The dexamethasone ophthalmic insert Dextenza (Ocular Therapeutix) is a new and exciting option for management of postoperative inflammation (Figure 2). Inserted in the operating room at the time of cataract surgery, the 0.4-mm dissolvable intracanalicular plug delivers a taper of steroid over the course of a month in the 4-3-2-1 dosing familiar to many surgeons. The plug is tagged with fluorescein for easy identification and monitoring at the slit lamp during postoperative visits. In the rare case of steroid-induced ocular hypertension, the punctal plug can be easily removed in clinic by massaging the plug out of the punctum.
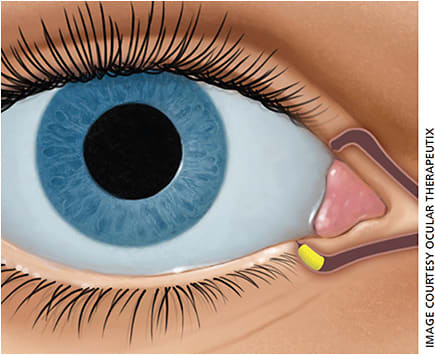
Dexycu
Dexycu (dexamethasone intraocular suspension 9%, EyePoint Pharmaceuticals) is another novel delivery tool that can simplify the cataract surgery journey for patients (Figure 3). Injected as a depot intracamerally behind the iris following IOL insertion and viscoelastic removal, Dexycu offers sustained-release dexamethasone over the course of one month and provides a continual, decreasing dose of steroid without drops following surgery. While this depot can migrate from behind the iris and into the anterior chamber, sometimes causing visually significant floaters or corneal edema, these are typically self-limited and resolve as the drop dissolves.
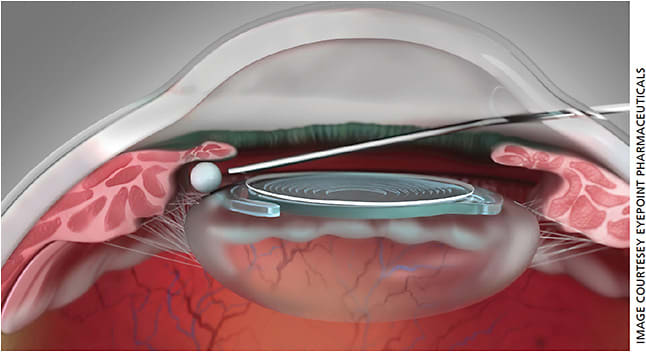
These new drop-sparing options for steroid delivery not only offer patients more independence and ease in the perioperative period, but they can also help decrease toxicity to the ocular surface by minimizing preservatives.
SIMPLIFYING THE JOURNEY
All of the exciting, developing technologies for drug delivery, such as drops and novel delivery mechanisms, aim to improve patient outcomes and comfort and simplify the months-long process of drops that patients have been accustomed to expect from cataract surgery. No single regimen is perfect for all patients, but our expanded toolbox of delivery mechanisms and agents allows us to customize each patient’s treatment to their needs.
We now have more delivery options available to patients who do not wish to, or are not able to, instill eyedrops. We also have better pharmacologic agents in drop form that are gentler on the ocular surface and require less frequent dosing than previously available. As technology continues to expand, so will our ability to further optimize the surgery and postoperative process for our patients. OM
REFERENCES
- Aptel F, Colin C, Kaderli S, et al. Management of postoperative inflammation after cataract and complex ocular surgeries: a systematic review and Delphi survey. Br J Ophthalmol. 2017;101:1-10.
- Laursen SB, Erichsen JH, Holm LM, Kessel L. Prevention of macular edema in patients with diabetes after cataract surgery. J Cataract Refract Surg. 2019;45:854-869.
- Kessel L, Tendal B, Jorgensen KJ, et al. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: a systematic review. Ophthalmology. 2014;121:1915-1924.
- Kim SJ, Schoenberger SD, Thorne JE, et al. Topical nonsteroidal anti-inflammatory drugs and cataract surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:2159-2168.
- Korenfeld MS, Silverstein SM, Cooke DL, Vogel R, Crockett RS; Difluprednate Ophthalmic Emulsion 0.05% (Durezol) Study Group. Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. J Cataract Refract Surg. 2009;35:26-34.
- Fong R, Silverstein BEE, Peace JH, Williams JI, Vittitow JL. Submicron loteprednol etabonate ophthalmic gel 0.38% for the treatment of inflammation and pain after cataract surgery. J Cataract Refract Surg. 2018;44:1220-1229.
- Shopf L, Enlow E, Popov A, Bourassa J, Chen H. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol Ther. 2014;3:63-72.
- Nassiri S, Hwang FS, Kim J, et al. Comparative analysis of intravitreal triamcinolone acetonide-moxifloxacin versus standard perioperative eyedrops in cataract surgery. J Cataract Refract Surg. 2019;45:760-765.









