Controlling and reducing IOP is the only way to reduce glaucoma progression and damage — at least to date. However, other risk factors and diagnostic testing including optic disc blood flow and visual field status have been identified to help further identify patients at higher risk.
Researchers are finding the value in new concepts such as corneal biomechanics (in particular, corneal hysteresis [CH]) and advanced imaging such as optical coherence tomography (OCT). Couple those with integrated telemedicine interventions for screening, and it’s hopeful the number of people with suspicious optic nerves, ocular hypertension or unusual retinal pathology can be more readily identified before vision loss occurs.
Here is an update on these glaucoma diagnostic technologies.
CORNEAL HYSTERESIS
Overview
“CH has made a significant impact on my ability to better diagnose and follow my glaucoma patients,” says Inder Paul Singh, MD, The Eye Centers of Racine and Kenosha (Wisconsin).
CH is the difference between the pressure at which the cornea bends inward during an airjet applanation and the pressure at which it bends out again, as determined by an infrared laser during an IOP measurement. This appears to quantify a biomechanical property of the cornea relating to its elasticity. CH should be thought of as the “shock absorber” of the eye, Dr. Singh says. Its value reflects the ability of the corneal tissue (and the rest of the eye) to absorb and dissipate energy.
In healthy eyes, CH is somewhere near 10 mm Hg, with a strong correlation between the eyes. In patients with primary open-angle glaucoma (POAG), however, CH is often lower (somewhere around 8-9 mm Hg or lower). Likewise, in healthy eyes there is a strong positive correlation between CH and central corneal thickness (CCT), but in glaucoma patients that correlation is much weaker.1
Research
Although landmark studies such as the Ocular Hypertension Treatment Study proved corneal thickness is an independent risk factor for glaucoma progression,2 many other studies have shown CH to be an even greater risk factor for glaucoma than CCT.1,3,4 “Eyes with lower CH cannot tolerate the IOP fluctuations as readily as those with normal CH levels,” Dr. Singh says, adding that the higher the IOP, the lower the CH tends to be (eyes with normal tension glaucoma also have lower CH levels compared to healthy eyes). Healthy eyes (or those with higher CH levels) can likely tolerate the IOP spikes more readily than those with lower CH. (Congdon et al. showed that for each mm Hg increase in CH, the rate of glaucoma progression declined by 20%.5) Further, both variables decline with age. One study found that when IOP was elevated, the optic nerve in patients with a high CH bowed back more than the optic nerve in people with lower hysteresis.6
CH may be used to predict optic nerve head susceptibility, Dr. Singh says, and may be a means of determining glaucoma progression. Medeiros et al demonstrated that baseline CH has a significant effect on the rate of visual field progression over time in patients with glaucoma.3 They found glaucomatous eyes with a CH ≥10 mm Hg did not have any rapidly progressive visual field loss; however, in the eyes with a CH <10 mm Hg, several cases of rapid progression occurred. In eyes with lower CH, the impact of IOP was significantly greater than in eyes with higher CH. In their multivariate model, CH was more than three times more commonly associated with increased rate of visual field progression than CCT.
CH is measured with the Ocular Response Analyzer (Reichert) or the Corvis ST (Oculus).
Determining a treatment plan
Dr. Singh says that CH impacts his treatment decisions.
“Hysteresis drops when the IOP gets very high,” he explains. “That makes sense because an eye that has a high pressure is already under stress; it’s already used up its ability to absorb energy or pressure. That eye, with its reduced flexibility, may be more susceptible to nerve damage. Conversely, when you lower the pressure, hysteresis increases; you’ve taken some stress out of the system. Now it has more absorbing capability again.”
So, a patient with ocular hypertension (OH) and a low CH, for example, warrants treatment more often than not. “But, if a patient comes in with OH and normal or high CH, I oftentimes won’t treat them, but I will continue to observe and monitor based solely on the CH measurement,” he says.
CH can be particularly useful in glaucoma suspects, he adds. “If visual fields are suspicious or if the eye is cupped and there’s a lower CH in that eye, that tells me the eye is at risk of progression and should not just be observed — or at least be observed more frequently.”
Often, the faster progressing eye has a lower CH than the more stable eye. Some glaucoma specialists also suggest closer follow-up and imaging the optic nerve, getting OCT and visual field data on each visit as well to help confirm progression. In addition, Dr. Singh says, CH was recently shown to decline at a faster rate in POAG compared to normal eyes.7
OCT-A
Overview
The most recent Ophthalmic Technology Assessment from the AAO8 noted structural glaucomatous damage to the retinal nerve fiber layer and optic nerve can be detected by spectral-domain OCT.8 In advanced cases of glaucoma, however, there is a recognized floor effect of OCT technology, where the increasing severity of disease has little to no effect on the imaging parameters. Because of these limitations, “OCT is not the best method to detect changes in advanced glaucoma.”8
OCT angiography (OCT-A) is a newer noninvasive modality “that detects blood flow through the motion contrast generated by red blood cells,”9 meaning it can detect blood vessel loss around the optic nerve and can distinguish vascular layers. Because it can provide an overview of the health of the retinal vasculature, it has the potential to help both diagnose and manage glaucoma (Figures 1 and 2). Clinically, the scans take only a few seconds, it is fairly easy to perform in patients who could have issues with cooperation, and no patient input is required so it is objective and repeatable. This means the technology can be easily incorporated into each patient visit.

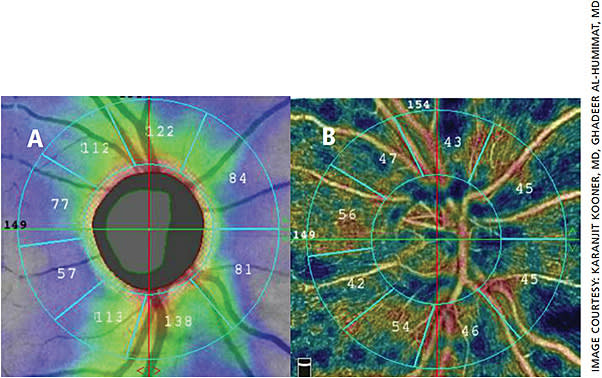
Research
A recent literature review of the technology found OCT-A has high repeatability and reproducibility, with the coefficient of variation staying below 7% in each of the three algorithms used.9 Most commercially available OCT-A systems use a split-spectrum amplitude-decorrelation algorithm, although there are others.
OCT-A allowed researchers to determine that lowering IOP surgically (via trabeculectomy) does not reverse the vascular loss. Some researchers are using OCT-A in various studies on the pathophysiology of glaucoma, which, if successful, may allow clinicians to personalize treatments. Whether those studies will be successful may not be known for years, given the relative slow state of disease progression.
There are limitations with using OCT-A. For example, motion artefacts and projection artefacts are common, rendering some images suboptimal in quality.10
Ready for “prime time?”
According to Grace M. Richter, MD, MPH, USC Roski Eye Institute in Los Angeles, OCT-A “will supplement current glaucoma diagnostic tools to aid in the early detection of glaucoma … OCT-A may elucidate the role of vascular pathophysiologic features in glaucoma.”11 Eyes with glaucoma have reduced ocular blood flow (OBF), “but we do not know whether reduced OBF is a cause or the simple result of glaucomatous optic neuropathy,” Dr. Richter says.
OCT-A is a promising tool, she adds, which may “even allow detection of salvageable retinal ganglion cells for rescue therapies.”
TELEMEDICINE
Overview
According to BrightFocus Foundation, 2.7 million Americans are affected by open-angle glaucoma. These numbers are estimated to rise to more than 7 million by 2050.12 Also on the rise is a potential shortage of ophthalmologists. With a recent U.S. Health Resources and Services Administration report estimating a shortage of 6,180 ophthalmologists by 2025,13 alternative means to reach these patients will be necessary.
One method that has gained traction is telemedicine, particularly in underserved and rural/remote areas as the technology allows increased access to health care. Technologies that implement a remote screening system for glaucoma allow non-specialists to detect glaucomatous changes in primary care or community settings. Even though Medicare policies include glaucoma screenings, it is well documented that there is poor follow-up adherence after the initial screening.14
Research
The Philadelphia Telemedicine Glaucoma Detection and Follow-up Study is a Centers for Disease Control-funded prospective randomized controlled trial that compares a social-worker intervention to address individual and systemic barriers to ophthalmology care vs. usual care.12 In that study, 906 people (553 female) consented and attended Visit 1 (at primary-care offices and Federally Qualified Health Centers). Of these, 356 participants (39.3%) were graded with a normal image. Using image data from the worse eye, 333 (36.8%) were abnormal and 155 (17.1%) were unreadable. A total of 258 (28.5%) had a suspicious nerve, 62 (6.8%) had ocular hypertension, 102 (11.3%) had diabetic retinopathy and 68 (7.5%) had other retinal abnormalities.
These findings support glaucoma screening via telemedicine within high-risk populations and referral to an eye-care provider for patients with unreadable fundus images or IOP greater than 21 mm Hg, the study authors noted. The researchers said “we believe that teleophthalmology, in conjunction with IOP measurements and clinical and demographic information, can increase the effectiveness of glaucoma screenings, allow for earlier diagnosis of glaucoma, improve access to care and treatment for glaucoma patients.” The study is ongoing.
Many uses
With tele-glaucoma, the use of devices such as automated perimetry, tonometry, corneal pachymetry, etc., generate digital outputs that can be transferred electronically and are portable.
Beyond traditional diagnostics, smartphones can take high-resolution fundus and optic nerve photos using various adapters (iExaminer, Welch Ally; iNview, Volk; the D-Eye, D-EYE). Home-based screening through the use of virtual reality (VR) tools is gaining in popularity as well, and some software programs can be used with existing VR programs without the need for a computer. Devices such as the iCare HOME allow patients to self-monitor their IOP. With patients having the ability to play a key role in their care by self-monitoring, compliance to treatment regimens may improve as well. OM
REFERENCES
- De Moraes CV, Hill V, Tello C, Liebmann JM, Ritch R. Lower Corneal Hysteresis Is Associated with More Rapid Glaucomatous Visual Field Progression. J Glaucoma 2012;21:209-13.
- Kass MA, Gordon MO, Gao F, et al. Delaying Treatment of Ocular Hypertension: The Ocular Hypertension Treatment Study. Arch Ophthalmol 2010;128:276-87.
- Medeiros FA, Meira-Freitas D, Lisboa R, Kuang TM, Zangwill LM, Weinreb RN. Corneal Hysteresis as a Risk Factor for Glaucoma Progression: A Prospective Longitudinal Study. Ophthalmology 2013;120:1533-40.
- Haseltine SJ, Pae J, Ehrlich JR, Shammas M, Radcliffe NM. Variation in Corneal Hysteresis and Central Corneal Thickness among Black, Hispanic and White Subjects. Acta Ophthalmol 2012;90:e626-31.
- Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. Central Corneal Thickness and Corneal Hysteresis Associated with Glaucoma Damage. Am J Ophthalmol 2006;141:868-75.
- Wells AP, Garway-Heath DF, Poostchi A, Wong T, Chan KC, Sachdev N. Corneal Hysteresis but Not Corneal Thickness Correlates with Optic Nerve Surface Compliance in Glaucoma Patients. Invest Ophthalmol Vis Sci 2008;49:3262-8.
- Hussnain SA, Alsberge JB, Ehrlich JR, Shimmyo M, Radcliffe NM. Change in Corneal Hysteresis over Time in Normal, Glaucomatous and Diabetic Eyes. Acta Ophthalmol 2015;93:e627-30.
- Chen TC, Hoguet A, Junk AK, et al. Spectral-Domain Oct: Helping the Clinician Diagnose Glaucoma: A Report by the American Academy of Ophthalmology. Ophthalmology 2018;125:1817-1827.
- Van Melkebeke L, Barbosa-Breda J, Huygens M, Stalmans I. Optical Coherence Tomography Angiography in Glaucoma: A Review. Ophthalmic Res 2018;60:139-151.
- Wan KH, Leung CKS. Optical Coherence Tomography Angiography in Glaucoma: A Mini-Review. F1000Res 2017;6:1686.
- Richter GM. The Promise of Optical Coherence Tomography Angiography in Glaucoma. Ophthalmology 2017;124:1577-1578.
- (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3401269/
- (https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/surgical-specialty-report.pdf )
- Hark LA, Katz LJ, Myers JS, et al. Philadelphia Telemedicine Glaucoma Detection and Follow-up Study: Methods and Screening Results. Am J Ophthalmol 2017;181:114-124.








