As we continue to see advancements in IOL technology, including extended depth of focus (EDOF) IOLs, multifocal IOLs (MFIOLs) or astigmatism-correcting IOLs, our ability to mimic the natural crystalline lens has driven better refractive outcomes and patient satisfaction. This, in turn, has created an increased patient demand for improved visual functionality following cataract surgery. However, these advanced technology IOLs are not without drawbacks, and the visual-demand dilemma is compounded in patients with additional pre-existing ocular pathology, such as glaucomatous changes.1-4
DYSFUNCTIONAL LENS AND GLAUCOMA
Results from the Framingham Eye Study and related reports5 demonstrate that both cataracts and glaucoma increase with age. The term “dysfunctional lens syndrome” has been applied to describe progressive changes to the crystalline lens, including loss of accommodation (presbyopia), decreased contrast sensitivity, increased higher-order aberrations and light scatter. Additionally, contrast sensitivity plays an important role in perimetric visual field testing and glaucomatous visual complaints in general. There is no shortage of data to demonstrate that visual field performance decreases as contrast sensitivity decreases. The role of contrast sensitivity has also been corroborated by questionnaires that reveal that despite patients having excellent visual acuity measured in office, the quality of that vision may be reduced.6
Reduced contrast sensitivity has been well documented in advanced technology IOLs,7 specifically compared to monofocal IOLs, so their use in glaucoma patients warrants significant preoperative discussions. Moreover, not all glaucoma types are created equal. The surgeon has specific considerations to keep in mind when developing a surgical plan for patients with glaucoma, considerations that may or may not entirely affect the patient’s final refractive outcome.
TORIC IOLS
Toric (or astigmatism-correcting) IOLs provide a means of counteracting the shape of a patient’s cornea without surgically manipulating the cornea. This astigmatism reduction not only lessens the strength of any possible postoperative spectacle prescription, it also has the opportunity to reduce corrective lens burden during follow-up glaucoma testing.
Brown et al performed a retrospective case study of 126 eyes implanted with toric IOLs in varying types/grades of glaucoma.8 Their results showed that 99.2% (125/126) of eyes had a corrected distance visual acuity (CDVA) of 20/25 or better. The refractive cylinder was reduced from 1.47 D (SD±1.10) preoperatively to 0.31 D (SD±0.37) postoperatively — a statistically significant change.
The researchers included patients with varying degrees of visual field loss and found that even patients with profound visual field defects experienced improvement in uncorrected distance visual acuity (UCDVA) and reduction of postoperative refractive cylinder.
This review, however, did have a paucity of patients diagnosed with pseudoexfoliation syndrome (PXF). We discuss the specifics of PXF later in this article. It would seem reasonable to recommend toric IOLs for glaucoma patients undergoing cataract surgery alone, as long as preoperative biometry testing reveals consistent keratometry readings. However, studies have shown that eyes undergo significant structural changes following either trabeculectomy or glaucoma drainage device implantation. The reports have shown anywhere from a 0.1- to 0.9-mm decrease in axial length following glaucoma surgeries, though this did not appear to have a statistically significant effect on mean refractive outcome.9,10
Tzu and colleagues studied the refractive outcomes of combined glaucoma (either trabeculectomy or glaucoma drainage device implantation) and cataract surgery and found that 74% (32/43) of eyes fell within a refractive outcome spherical equivalent range of -1.00 D to +0.50 D at 3 to 6 months follow-up.11 This was coupled with an induced with-the-rule astigmatism of 1.31 D (SD±0.86), regardless of glaucoma surgical approach. This is certainly a consideration worthy of extensive perioperative discussion on visual outcomes.
MULTIFOCAL IOLS
MFIOLs (bifocal, trifocal, diffractive, refractive) are a beneficial option if patients desire near-complete spectacle independence. However, they, too, are not without consequences: Transient postoperative glare, halos and reduced contrast sensitivity have been reported.12 Because reduced contrast sensitivity is of primary concern in glaucoma patients, the surgeon may be initially wary of offering a MFIOL to a glaucoma patient.
Souza and colleagues compared the AcrySof ReSTOR apodized diffractive IOL to the AcrySof SA60AT monofocal IOL (Alcon) and found that, despite suffering from reduced monocular photopic contrast sensitivity, patients implanted with the ReSTOR had comparable UDVA and CDVA, comparable quality-of-life scores and statistically significant better near vision when compared to the SA60AT group.13
Similarly, Kamath et al14 studied a zonally progressive refractive MFIOL, the Array (AMO, Allergan), implanted in eyes with additional ocular pathology compared to a monofocal IOL (AMO SI-40NB). Twelve patients with the diagnosis of glaucoma or ocular hypertension received the monofocal implant, while 11 eyes with glaucoma and six eyes with ocular hypertension were implanted with the MFIOL. Although the Array MFIOL showed decreased contrast sensitivity at lower contrast levels, no statistically significant differences were demonstrated between the two groups, apart from better uncorrected near vision in the MFIOL group.
Kohnen et al15 studied the PanOptix trifocal IOL (AcrySof IQ PanOptix; Alcon). This IOL combines the traditional trifocal design with a quadrifocal design to create a new technology called Enlighten, which provides a near focal point at 40 cm and an intermediate focal point at 60 cm, with an 88% light transmission profile. Once again, contrast sensitivity was reduced compared to a similar monofocal model (AcrySof Natural SN60AT; Alcon), although near and intermediate visual acuity was enhanced compared to the monofocal group.
The fact that visual field changes can occur after cataract surgery is an especially important consideration when it comes to patients implanted with a MFIOL. Aychoua et al16 evaluated Humphrey visual field (Humphrey Field Analyzer [Zeiss]) SITA 30-2 testing in patients implanted with MFIOLs (Tecnis ZM900, J&J Vision; Zeiss 809M, AT LISA, Carl Zeiss Meditec) and monofocal IOLs (Tecnis ZC9003; Johnson & Johnson Vision) compared to phakic patients. They found that with stimulus III testing, the mean deviation for the MFIOL group was 2.40 dB lower compared to the phakic group. The monofocal group only showed a 0.32 dB drop in mean deviation compared to the phakic group, leading the authors to speculate that the drop in mean deviation must be related to the MFIOL rather than the pseudophakic status itself.
In corroboration, Farid et al17 studied the impact of Humphrey visual field 10-2 testing following implantation with different types of MFIOLs: Tecnis ZMB00, Tecnis ZMA00 (Johnson & Johnson Vision) and AcrySof IQ ReSTOR SN6AD1 (Alcon). They found the MFIOL group had an average mean deviation of -2.84 dB (SD±2.32) compared to -0.97 dB (SD ±1.58) in the monofocal group. This decrease in mean deviation did not improve in the MFIOL group, even 6 months after cataract surgery.
This is of special concern for patients with advanced-stage glaucoma, as the decrease may interfere with monitoring the remaining central island of vision. It is important to obtain new “baseline” glaucoma testing following cataract surgery.
EXTENDED DEPTH OF FOCUS IOLS
EDOF IOLs (Tecnis Symfony, Johnson & Johnson Vision) create an elongated focus with 92% light transmission to enhance intermediate and near visual performance while minimally affecting distance performance. The EDOF IOLs are designed to provide less glare, halos and less contrast sensitivity loss compared to diffractive MFIOLs. Studies regarding visual outcomes and patient satisfaction following EDOF implantation have been favorable, but the lens design still carries the risk of postoperative dysphotopsias.18 Despite the lack of published data to support the use of EDOF IOLs in patients with glaucoma, we have observed that these lenses can be used with caution in patients with well-controlled ocular hypertension and mild glaucoma.
Although advanced technology IOLs have their drawbacks, surgeons should still consider their use if a patient desires an outcome with the possibility of spectacle independence. It is up to the individual ophthalmologist to provide the appropriate and adequate patient counseling in the perioperative period.
PSEUDOACCOMMODATION AND ACCOMMODATIVE IOLS
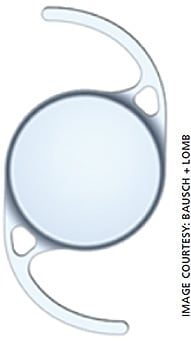
“Pseudoaccommodation” describes patients implanted with an emmetropic-targeting monofocal IOL who have unexpected improvement in near visual acuity. This phenomenon has been linked with the depth of focus on an eye19,20 as well as utilizing the patient’s corneal aberrations to essentially produce areas of multifocality within the eye. One monofocal platform, the enVista (MX60E) and its toric version (MX60T, Bausch + Lomb; Figure 1) are aberration-free monofocal IOLs that maintain the corneal aberrations. The result is slight multifocality without the significant dysphotopsia profile of the MFIOLs.
In the pursuit of mimicking the crystalline lens function, accommodative IOL (AIOL) design has become of increasing interest. AIOLs, such as the Crystalens (B + L), Lumina AIOL (Akkolens International) and Akkommodative ICU lens (HumanOptics AG) have demonstrated comparable CDVA and contrast sensitivity to monofocal IOLs, with varying reports on the intermediate and near vision functionality of these AIOLs.21 The Crystalens is the only of these IOLs currently approved in the United States. Dysphotopsia complaints, although not as prevalent as with MFIOLs, were still reported with AIOLs. However, we lack data on AIOLs and concomitant eye disease such as glaucoma.
SPECIAL CONSIDERATIONS
There are two in particular to keep in mind:
- Defocus curves. An important aspect of MFIOL/EDOF selection for cataract surgery patients is the defocus curve of the selected IOL. Broadly, a defocus curve describes how the IOL performs when placed under refractive circumstances outside of the BCVA, essentially revealing the range of functional vision. This is an important metric when considering the visual needs of our patients. Most MFIOL/EDOF IOLs have ample data surrounding their defocus curves, so these data are easily obtainable.
- Pseudoexfoliation syndrome (PXF). PXF has been well documented as a leading cause of open-angle glaucoma.22 The deposition of extracellular material that occurs within an eye with PXF leads to several concerns, including operative (poor pupillary dilation, zonular complex instability) and postoperative (IOL destabilization/subluxation, IOP elevation, increased posterior capsular opacification) issues.23,24 If the surgeon does not take care to mitigate these issues intraoperatively, the result may be a prolonged postoperative course, with the possible need for further surgical intervention. The surgeon must consider the higher rates of IOL destabilization/subluxation in patients with PXF. For an advanced technology IOL to function optimally, excellent centration and a clear optic axis are required. Any decentration or opacification of an advanced technology IOL has been shown to increase higher-order aberrations, which can significantly degrade a patient’s visual acuity and quality of life.25,26
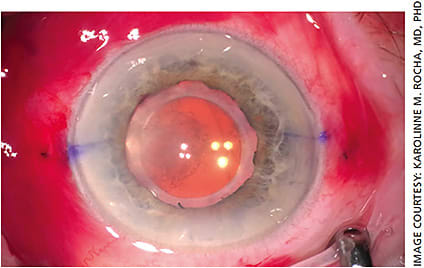
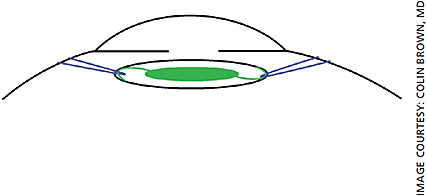
For our patients with PXF, we generally recommend a three-piece acrylic, hydrophobic monofocal IOL. New one-piece IOL designs have emerged that may give the surgeon more peace of mind when implanting into an eye with PXF. The enVista IOLs (Figure 1) are designed with a “fenestrated haptic,” which is essentially an eyelet on the haptic arm near the optic-haptic junction. This eyelet may be utilized for suture fixation of the dislocated IOL complex. Tecnis IOLs (Figure 2) have a notch near the optic-haptic junction, which would be the preferred location to place a fixation suture using such maneuvers as the McCabe belt loop technique (Figures 3A and 3B).

In the event that an advanced technology IOL is implanted and the patient cannot tolerate the side effects, there may be a less invasive way to remedy the issue than IOL exchange. Femtosecond laser technology can be used to correct residual refractive error of implanted hydrophilic or hydrophobic acrylic IOLs.27 This not-yet-approved technology, refractive index shaping (Perfect Lens), uses a femtosecond laser to change the optical properties and refractive index of the implanted lens; it can change a monofocal IOL into a multifocal IOL and vise versa. This can be important when treating glaucoma patients, as those who are intolerant to the MFIOL/EDOF dysphotopsias or have significant progression of their glaucoma postoperatively can have their IOL altered with an in-office procedure.
A BRIGHTER FUTURE
In the end, IOL selection remains in the domain of proper patient counseling. As new advanced technology IOLs are developed, we need to study how they affect clinical testing and patient quality of life in eyes with comorbid conditions such as glaucoma. Toric IOLs remain an excellent option for all glaucoma types for possibly reducing spectacle correction while not interfering with future glaucoma testing. MFIOL/EDOF lenses remain a good option for ocular hypertension and mild glaucoma patients, but their use in more advanced stages of glaucoma where contrast sensitivity and visual field testing can be affected is not recommended.
One must continue to exercise caution in PXF patients, as discussed above. As new accommodative IOL designs are developed, further research into their utility in glaucoma patients must follow. OM
REFERENCES
- Teichman JC, Ahmed II. Intraocular lens choices for patients with glaucoma. Curr opin ophthalmol. 2010;21:135-143.
- Kumar BV, Phillips RP, Prasad S. Multifocal intraocular lenses in the setting of glaucoma. Curr opin ophthalmol. 2007;18:62-66.
- Braga-Mele R, Chang D, Dewey S, et al. Multifocal intraocular lenses: relative indications and contraindications for implantation. JCRS. 2014;40:313-322.
- Iancu R, Corbu C. Premium intraocular lenses use in patients with cataract and concurrent glaucoma: a review. Mædica. 2013;8:290-296.
- Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA et al (1980) The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24:335-610.
- Hawkins AS, Szlyk JP, Ardickas Z, Alexander KR, Wilensky JT. Comparison of contrast sensitivity, visual acuity, and Humphrey visual field testing in patients with glaucoma. J Glaucoma. 2003;12:134-138.
- de Vries NE, Nuijts RM. Multifocal intraocular lenses in cataract surgery: literature review of benefits and side effects. JCRS. 2013;39:268-278.
- Brown R, Zhong L, Bozeman C, Lynch M. Toric intraocular lens outcomes in patients with glaucoma. JCRS. 2015; 31: 366-372.
- Pakravan M, Alvani A, Esfandiari H, Ghahari E, Yaseri M. Post-trabeculectomy ocular biometric changes. Clin Exp Optom. 2017;100:128-132.
- Francis BA, Wang M, Lei H, et al. Changes in axial length following trabeculectomy and glaucoma drainage device surgery. BJO. 2005;89:17-20.
- Tzu JH, Shah CT, Galor A, Junk AK, Sastry A, Wellik SR. Refractive outcomes of combined cataract and glaucoma surgery. J Glaucoma. 2015;24:161-164.
- Breyer DRH, Kaymak H, Ax T, Kretz FTA, et al. Multifocal intraocular lenses and extended depth of focus intraocular lenses. APJO. 2017;6:339-349.
- Souza CE, Muccioli C, Soriano ES, et al. Visual performance of AcrySof ReSTOR apodized diffractive IOL: a prospective comparative trial. Am J Ophthalmol. 2006;141:827-832.
- Kamath GG, Prasad S, Danson A, Phillips RP. Visual outcome with the array multifocal intraocular lens in patients with concurrent eye disease. JCRS. 2000;26:576-581.
- Kohnen T, Herzog M, Hemkeppler E, et al. Visual performance of a quadrifocal (trifocal) intraocular lens following removal of the crystalline lens. Am J Ophthalmol. 2017;184:52-62.
- Aychoua N, Montolio FGJ, Jansonius NM. Influence of multifocal intraocular lenses on standard automated perimetry test results. JAMA Ophthalmol. 2013;131:481-485.
- Farid M, Chak G, Garg S, Steinert RF. Reduction in mean deviation values in automated perimetry in eyes with multifocal compared to monofocal intraocular lens implants. Am J Ophthalmol. 2014;158:227-231.
- Liu J, Dong Y, Wang Y. Efficacy and safety of extended depth of focus intraocular lenses in cataract surgery: a systematic review and meta-analysis. BMC Ophthalmol. 2019;19:1-10.
- Nakazawa M, Ohtsuki K. Apparent accommodation in pseudophakic eyes after implantation of posterior chamber intraocular lenses: optical analysis. Invest Ophthalmol Vis Sci. 1984;25:1458-1460.
- Pallikaris IG, Kontadakis GA, Portaliou DM. Real and pseudoaccommodation in accommodative lenses. J Ophthalmol. 2011;2011:1-8.
- Alió JL, Alió JLB, Vega-Estrada A. Accommodative intraocular lenses: where are we and where we are going. Eye Vis. 2017;4:16.
- Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141:921-937.
- Sangal N, Chen TC. Cataract surgery in pseudoexfoliation syndrome. Semin Ophthalmol. 2014;29(5-6):403-408.
- Fontana L, Coassin M, Iovieno A, Moramarco A, Cimino L. Cataract surgery in patients with pseudoexfoliation syndrome: current updates. Clin Ophthalmol. 2017;11:1377-1383.
- Elgohary MA, Beckingsale AB. Effect of posterior capsular opacification on visual function in patients with monofocal and multifocal intraocular lenses. Eye. 2006;22:613-619.
- Soda M, Yaguchi S. Effect of decentration on the optical performance in multifocal intraocular lenses. Ophthalmologica. 2012;227:197-204.
- Sahler R, Bille JF, Enright S, Chhoeung S, et al. Creation of a refractive lens within an existing intraocular lens using a femtosecond laser. JCRS. 2016;42:1207-1215.










