Prior to the approval of corneal collagen crosslinking (CXL) in the United States in 2016, we had no effective treatment to limit the advancement of keratoconus, a progressive ectatic disorder of the cornea. “Epi-off,” approved by the FDA only 4 years ago, offers a way to stop, and even reverse, progression that leads to distorted vision, corneal transplantation and vision loss. But epi-off has not been without disadvantages of its own. A new variation on CXL, “epi-on,” may deliver the benefits of epi-off while avoiding its drawbacks and risks.
KERATOCONUS
A review
Keratoconus has been described as a progressive ectatic disorder of the cornea, usually beginning in adolescence. It may start as mild visual changes then progress to severe vision loss.1-3
Without treatment, this may progress from astigmatism that is correctable with spectacles to irregular astigmatism that requires rigid contact lenses and eventual severe disease requiring corneal transplantation. It has been estimated that 11% to 27% of patients with keratoconus would eventually require corneal transplantation due to severe thinning, ectasia or scarring that rendered these patients uncorrectable optically.1,4,5
But even if a patient has a graft and obtains excellent vision, that does not always mean the procedure is a success. Most grafts do not last forever, so young patients undergoing a graft may end up having additional grafts over the course of their lives. In addition, they are at risk for other surgical and postoperative complications, such as cataract, glaucoma and trauma.
A number of years ago, before CXL was available, I performed a transplant on an 18-year-old patient. He corrected to 20/20 very soon after surgery, but as is commonly the case with young patients he was very active and was hit in his eye, rupturing his wound. He was repaired in the OR and eventually obtained 20/20 vision again, but was lost to follow-up. When he returned, he had not been using his steroid drops and developed graft rejection. Again, he was treated and was able to obtain 20/20 vision.
Nevertheless, it is difficult to consider him a true success when we consider what he has been though already and what he will be facing in the future. He will likely have an early cataract and will always need to be monitored for glaucoma. Had we been able to crosslink this eye and avoid the graft, he would likely be much better off now.
CORNEAL CROSSLINKING BEGINNINGS
Development and U.S. approval
CXL, first studied in the late ‘90s, has been found to have a strengthening and stabilizing effect on the cornea.1,6,7 While this procedure has been available internationally for years, it was approved in the United States in 2016. The procedure involves saturating the cornea with a photosensitizer, riboflavin (vitamin B2), and then exposing the cornea to ultraviolet-A (UV-A) light. This photochemical treatment creates additional chemical bonds between the stromal collagen fibers by photopolymerization, which theoretically increases the stiffness. Following exposure to UV-A radiation, riboflavin acts as a photomediator as its molecules absorb energy, reach an excited state and subsequently produce free radicals to induce new chemical bonds.
The idea of using CXL to treat keratoconus patients was first described by Wollensak et al in 2003. This study found that all 23 treated eyes stopped progressing, and 16 of the eyes actually showed signs of regression.1,8 A pivotal 2017 trial of epithelium-off crosslinking found that treated eyes showed a flattening of maximum K value (Kmax) of 1.6 D, while the control eyes had an increase in Kmax of 1.0 D at 12 months for an overall difference of 2.6 D.1,9
Epi-off drawbacks and considerations
While epithelium-off crosslinking is FDA-approved and has gained significant momentum in the United States, it is not without risks. The removal of the epithelium has all of the drawbacks that we have long known, most significantly pain, infection and delayed wound healing. Many surgeons choose to apply a bandage soft contact lens at the end of the procedure to provide comfort and perhaps aid in wound healing. In addition, I have my patients apply antibiotic eyedrops for 1 week postoperatively. I also have them use a steroid drop for 4 weeks and a non-steroidal drop for the first day or two for pain.
It is imperative to make sure the epithelium heals in a timely fashion as well. While any treated cornea may develop haze following treatment, a prolonged defect from the procedure may increase this risk. In the pivotal trial, the haze tended to resolve over several months.1,9
In addition, there are other factors to consider with epithelium removal. The FDA approved this procedure in corneas with a minimum thickness of 400 µ. It is not uncommon for keratoconic corneas to be very thin, even less than 400 µ. During the traditional epi-off treatment, the cornea receives riboflavin eye drops for 30 minutes. During this time, due to exposure and desiccation, the cornea may thin significantly and fall below the 400-µ level. Just the removal of the epithelium prior to starting treatment may also thin the cornea by about 50 µ. Then, the hypertonic formulation of riboflavin, approved by the FDA, may cause further dehydration and thinning of the cornea. It is not known if treating a thin cornea will lead to endothelial damage, but it has been a concern for many.
Fortunately, we have mechanisms to deal with the thinning. One option is to leave the speculum out and allow the patient to close their eyes during the induction phase to limit desiccation. In addition, a hypotonic version of riboflavin is approved by the FDA. Applying the hypotonic drop to the cornea may allow significant swelling of the cornea to above the 400-µ level and then allow on-label treatment. In my experience performing hundreds of epi-off procedures, no matter how thin the cornea is I have yet to find one that was too thin to swell above 400 µ with the hypotonic riboflavin.
THE ALTERNATIVE: EPI-ON
What it is, how it differs
Because of these well-known drawbacks of removing the epithelium, epi-on, or epithelium sparing, procedures are also being investigated in the United States. Clinical trials are currently underway, including Avedro’s FDA Phase 3 trial. Epi-on offers numerous potential advantages. First, sparing the epithelium significantly decreases the discomfort and pain for the patient. This alone may be enough for many patients to desire this method. By leaving the epithelium intact, we may also decrease the risk of infection, as well as haze related to a slowly healing defect.
A closer look
While leaving the epithelium intact has obvious theoretical advantages, the option also comes with theoretical disadvantages. First and foremost, will the procedure be effective? There are three components that need to be present within the cornea for treatment to take effect: riboflavin, UV light and oxygen. The epithelium may limit penetration of all three components into the stroma, therefore limiting the effect of the treatment. The demarcation line seen on imaging following treatments, which represents depth of treatment in the cornea, may be shallower for epi-on. It is unknown what significance this finding has, both from a flattening perspective and a stability perspective.
Research shows that in room air, oxygen within the corneal stroma depletes almost immediately once UV light treatment begins — which means the treatment is being done in an anaerobic environment. It may then take minutes for the oxygen level to return to baseline once the light is turned off.6 Therefore, merely pulsating the light on and off during treatment may not allow enough time for the cornea to return to an aerobic environment.
This has led to the investigation of using supplemental oxygen at the time of treatment. A recent study of corneal oxygen concentration in pig eyes had some interesting findings. Without adding any supplemental oxygen, even with epi-off treatment, the oxygen concentration within the stroma dropped to anaerobic levels upon initiating treatment.
On the other hand, upon initiating treatment in a hyperoxic environment, the stromal oxygen level remained above the level it was when in room air prior to starting treatment. This may indicate that the stroma may remain adequately oxygenated for aerobic treatment when supplemental oxygen is used.10
Case report
A 17-year-old male with keratoconus presented for a second opinion, having been told that he needed a corneal transplant. On exam, his UCVA was 20/100 and his BSCVA was 20/60. He stated that he was contact lens intolerant. On slit lamp exam, while his cornea showed apical thinning, no scarring was present. On topography, he had a Kmax of 67.5 (Figure 1, pretreatment topography). Because his visual axis was clear, it was recommended that he undergo corneal crosslinking.
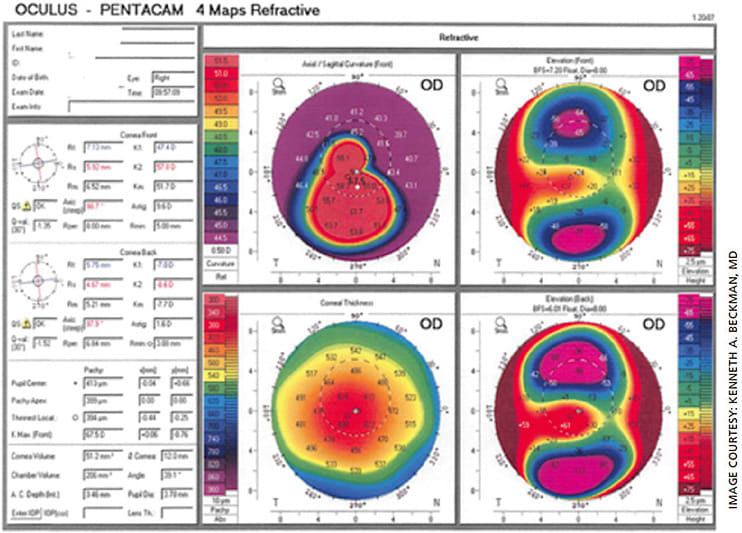
Eight months later, his UCVA was 20/30 and his BSCVA was 20/25. The Kmax on topography testing had decreased to 65.7 (Figure, 2, post treatment topography). Obviously, with his tremendous improvement in visual acuity, he did not require a transplant. In addition, the reshaping of his cornea meant he could more easily be fitted with a contact lens, but he chose not to wear contact lenses due to his excellent uncorrected vision (Figure 3).
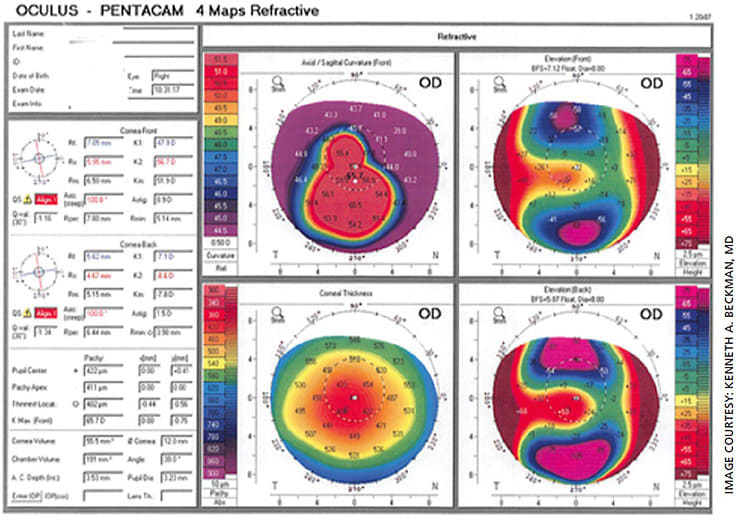
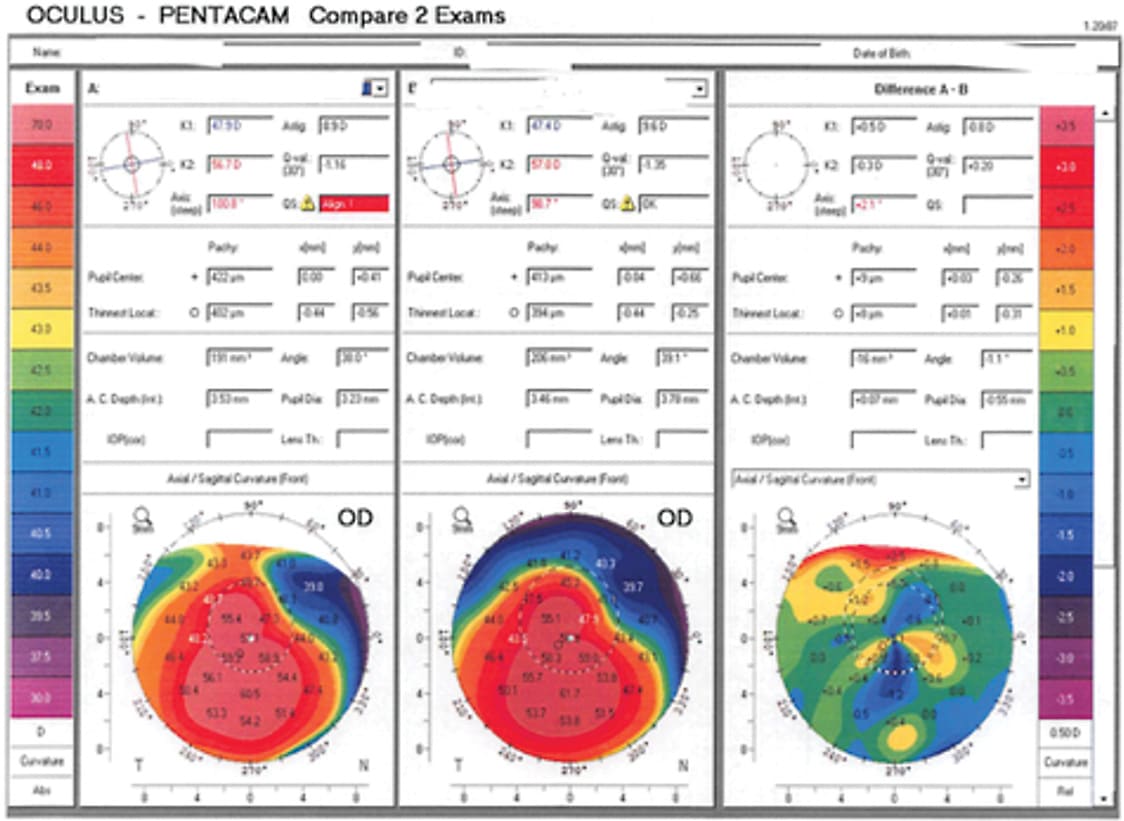
Ongoing clinical trial
These findings were the basis of the protocol Avedro used in its ongoing epi-on clinical trial. In this trial, Avedro has altered the traditional parameters used in the approved Dresden protocol to address the above-mentioned concerns with epi-on. First, the company created a new preparation of riboflavin, which penetrates epithelium better. Avedro also uses an oxygen mask to provide supplemental oxygen to the cornea in attempt to keep the treatment aerobic.
Finally, the company uses a higher intensity, shorter duration light, pulsating on and off, in hopes of improving light penetration and some oxygen reaccumulation during each off pulsation. While patient enrollment is complete, the trial is still ongoing. Other protocols are currently being used internationally and are under investigation in the United States as well.
In my opinion, if an epi-on protocol demonstrates efficacy similar to epi-off, it will quickly become the primary method used.
TREATMENT DECISIONS
When to use CXL
Patient selection for crosslinking, whether for epi-off or epi-on, has been confusing for many eye-care professionals. The treatment has been approved for “progressive” keratoconus, but the definition of progression is unclear. For clinical trials, inclusion criteria to define progression varies from one trial to another, as no true consensus exists. Criteria commonly used based on the Avedro label is an increase in Kmax of 1 D, an increase in cylinder on refraction of 1 D or a myopic shift on refraction of 0.5 D.11
However, because younger patients may progress much more rapidly, the notion of waiting for progression in very young patients may make some surgeons uncomfortable. I have seen teenagers progress rapidly within months, which leads me to opt for early intervention in these patients. In addition, because the treatment is best for slowing progression, rather than leading to regression, the ideal patients are those who still have excellent vision but are at risk to progress. The need to identify such patients early has led to an important adjustment in approach amongst eye-care gatekeepers. In the past, these patients were followed for years, given glasses and contacts, and eventually referred to a cornea specialist when they needed surgery. Now, thanks to education in this area, optometrists and comprehensive ophthalmologists are sending young patients for evaluation much earlier.
Another group that seems to be missed is the more advanced patient. In the past, patients who had poor visual acuity and could not tolerate a contact lens were immediately referred for transplant surgery. Even with the onset of crosslinking, there is still a misunderstanding that treatment will only stabilize a patient and not lead to improvement. Fortunately, we now know this is not the case. In my practice, unless the patient has a visually significant central scar, I always recommend CXL before jumping to transplant.
Better scleral lenses help
Because scleral lenses have improved so much and are so much easier to customize, almost any patient can be fit with a contact lens. If the patient does not have a central scar, no matter how deformed the cornea is, it is highly likely that the patient will obtain excellent vision if a contact lens can be fitted. Also, even if the patient is not contact-lens tolerant to begin with, the cornea frequently reshapes enough after treatment that they actually become contact-lens tolerant. It is important to remember that a high percentage of transplant patients eventually need contact lenses after surgery anyway, so if a graft can be avoided, it is always advantageous.
In addition, many patients show so much improvement — rather than mere stabilization — that they may not even need contact lenses following treatment. Countless patients have been sent to me for a second opinion after they were told they need a graft, only to find that they obtain outstanding vision after crosslinking.
TRANSPLANTS MAY BE A THING OF THE PAST
In summary, keratoconus is a progressive and potentially debilitating condition, but now there is a treatment that may limit progression. If we catch these patients before vision loss occurs, we may save countless patients from undergoing corneal transplants.
Further, just because a patient progresses to transplant level vision does not mean that he or she cannot benefit from crosslinking to prevent the need for transplantation. If their visual axis is optically clear, they may benefit from crosslinking and then subsequent scleral contact lens fit and avoid a graft altogether. OM
REFERENCES
- Beckman KA, Gupta PK, Farid M, Berdahl JP, et al, the ASCRS Cornea Clinical Committee. Corneal crosslinking: Current protocols and clinical approach. J Cataract Refract Surg. 2019; 45:1670-1679.
- Romero-Jimenez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010; 33:157-166.
- Ferdi AC, Nguyen V, Gore DM, Allan BD, Rozema JJ, Watson SL. Keratoconus natural progression: a systematic review and meta-analysis of11&thinsp 529 eyes. Ophthalmology. 2019; 126:935-945.
- Javadi MA, Motlagh BF, Jafarinasab MR, et al. Outcomes of penetrating keratoplasty in keratoconus. Cornea. 2005; 24:941-946.
- Mamalis N, Anderson CW, Kreisler KR, et al. Changing trends in the indications for penetrating keratoplasty. Arch Ophthalmol. 1992; 110:1409-1411.
- Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci. 2012; 53:2360-2367.
- Beshtawi IM, O’Donnell C, Radhakrishnan H. Biomechanical properties of corneal tissue after ultraviolet-A–riboflavin crosslinking. J Cataract Refract Surg. 2013; 39:451-462.
- Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A–induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003; 135:620-627.
- Hersh PS, Stulting RD, Muller D, the United States Crosslinking Study Group. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017; 124:1259-1270; erratum, 1878
- Hill J,Liu C, Deardorff P, Tavakol B, et al. Optimization of oxygen dynamics, UV-A delivery and drug formulation for accelerated epi-on corneal crosslinking. Curr Eye Res. 2020 Apr;45:450-458.
- Avedro label. https://int.avedro.com . Accessed April 27, 2020.









