Neurotrophic keratitis (NK) remains a very challenging problem for ophthalmologists. This degenerative corneal disease is marked by delayed epithelial healing due to impaired or altered corneal sensation. Chronically decreased corneal sensation leads to a clinical picture that is all too familiar to many of us: an appearance of chronic punctate staining, decreased corneal luster and possibly ulceration with epithelial defects and stromalysis. Addressing NK requires a chronic, comprehensive and systematic therapeutic approach. NK may cause visual compromise; if ineffectively or inadequately treated, the condition often leads to irreversible ocular surface damage and vision loss, sometimes profound in nature. Chronic changes may include fibrotic and vascular scarring. Occasionally, it may even lead to corneal perforation. Treating at earlier stages of the condition can be more straightforward and yield both better ocular surface health and visual function (Table 1).
| STAGE | SLIT LAMP FINDINGS |
|---|---|
| 1 | Punctate keratitis; epitheliopathy |
| 2 | Epithelial defect with intact stroma, ulceration without stromal melt |
| 3 | Epithelial defect with stromal ulceration, and loss due to stromalysis; leading to perforation |
Fortunately, although NK remains a very challenging condition, we have several therapeutic options in our toolbox that are efficacious.
THE CAUSES
While several conditions lead to NK, herpes simplex and herpes zoster are the classic causes.1 Topical medication toxicity, chronic ocular surface disease and long-standing dry eye (stain without pain) are other common conditions we see that lead to NK. Other contributing factors include ocular surgery, such as large limbal-relaxing incisions that interrupt the corneal nerve bundles as they enter the stroma from the peripheral cornea, LASIK flaps that disrupt the corneal nerves closer to the surface and contact lens overwear. We often do not associate diabetes mellitus as a frequent cause of corneal nerve impairment, despite the understood and common diabetic neuralgias and ulcers. Cerebrovascular accidents and neurosurgical procedures that affect the trigeminal nerve may also cause NK.2
FIRST STEP: TESTING SENSATION
It is critical to diagnose the underlying condition, whether you are testing with a Cochet-Bonnet esthesiometer, which can better identify “relative” or more mildly decreased corneal sensitivity (hypoesthesia), or using a cotton-tipped applicator pulled out a bit to create a more gentle cotton wisp at the tip or even dental floss (unused), to identify more complete loss of corneal sensitivity (anesthesia).3 Testing sensation over the involved area and comparing that to a mirror area in the fellow eye is important in the evaluation of these patients. It is essential to test before the application of a topical anesthetic; alternatively, specify the evaluation of corneal sensation be performed at a future visit, if topical anesthesia has already been applied.
TREATMENTS AT OUR DISPOSAL
Treatment options may vary depending on the stage of NK (Figures 1-3).4 The majority of NK patients will already be placed on preservative-free tears, gels or lubricant ointments, whether on the recommendation of the eye-care professional, who then referred them to a corneal specialist, or as an attempt at self-medication. Additional medical treatments include:
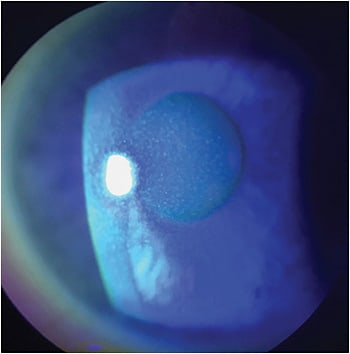
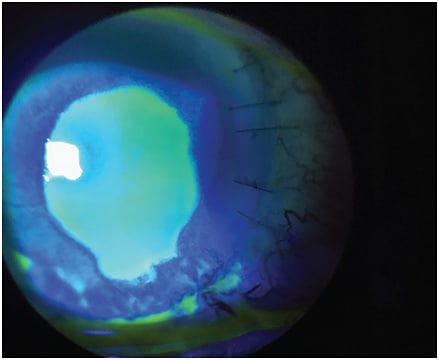
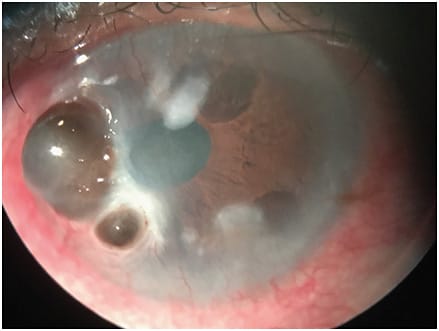
- Topical medications that may include a combination of antibiotics to prevent secondary infection and/or topical anti-inflammatory agents. Topical secretagogue agents should also be considered.
- Blood products in the form of autologous serum tears (50% is my preferred concentration), or one of the newer artificial tear preparations that include additional elements to promote ocular surface healing. These provide a variety of regenerative components, including vitamin A, fibronectin, growth factors and albumin.
- Topical retinoic acid ointments and topical cyclosporine or lifitegrast may have some benefit as adjunctive therapies.
- Therapeutic bandage contact lenses (typically silicone hydrogel contact lenses), which act as a barrier and promote healing passively by blocking the eyelid rubbing over the epithelial surface, allowing the adherence of the healing epithelial cells.
- Scleral contact lenses.
- Amniotic membrane (AM), whether self-retaining, inserted in the office or sutured in place, provides anti-inflammatory and anti-angiogenic factors while acting as a scaffold to help with epithelial regeneration. Several variations of AM are available, including the cryopreserved AM, which appears to preserve more of these important factors, or freeze-dried AM products.
- Punctal occlusion — temporary, extended or permanent — is an adjunctive therapy that may be used combined with any and all of the other treatments.
A tarsorrhaphy or conjunctival flap may be considered. They represent the previous gold standards for the treatment of severe NK cases, although they create an undesirable cosmesis for many patients due to the postop appearance. The lid closure may be temporary or permanent. It is typically achieved with sutures, but may also be performed with Botox or tissue glue, or intermittently with properly positioned tape or plastic.
More severe cases of NK may lead to corneal perforation, which would require a corneal glue patch/therapeutic contact lens if small or a therapeutic keratoplasty combined with a lateral tarsorrhaphy if large.4
NOVEL TECHNIQUES FOR NK
Surgical
Corneal neurotization is the process of surgically transferring a healthy donor nerve segment to a cornea with NK, for restoration of corneal nerve function. The first report of corneal neurotization was described in 2009 by Terzis et al,5 in which six patients with unilateral facial and trigeminal palsies underwent direct neurotization of the cornea using the contralateral supraorbital and supratrochlear branches of the ophthalmic division of the trigeminal nerve.
This novel technique requires invasive surgery and patience regarding the result: Corneal nerve regeneration can vary from several months to more than 2 years. Other reports have described using sural nerve grafts as well as the ipsilateral supraorbital nerve for grafting.6 This technique is highly specialized and only performed in a few centers throughout the United States, yet it is the only treatment that surgically reconnects nerve tissue back to the cornea.
Pharmaceutical
Oxervate (cenegermin-bkbj 20 mcg/ml, Dompé) is a novel medical treatment that addresses the root cause of NK by providing a recombinant form of human nerve growth factor (rhNGF) to stimulate corneal epithelial cell proliferation, differentiation and tear secretion.7,8 The active ingredient in Oxervate is a protein that is structurally identical to the naturally occurring nerve growth factor (NGF) produced by the human body.9 Endogenous NGF is a protein that is vital to the differentiation and maintenance of corneal nerves.10 The corneal nerves release neuromediators that provide trophic support to the ocular surface, stimulate wound healing and maintain anatomic integrity.3 These mediators in turn stimulate the epithelium to release neurotrophins, neuropeptides and growth factors like NGF and EGF to promote corneal nerve fiber maturation and health.
Tear secretion is critical in delivering these necessary trophic and growth factors to receptors on the lacrimal gland and to nerves on the ocular surface. NGF acts through specific high-affinity (ie, TrkA) and low-affinity (ie, p75NTR) nerve growth factor receptors in the anterior segment of the eye to support corneal and conjunctival innervation and integrity and to promote the homeostasis of the ocular surface dynamic.3,10
Oxervate efficacy and safety
We should evaluate the efficacy of Oxervate in relation to the severity of the conditions for which we choose to employ it. Our instinct as clinicians is to view any novel treatment with a bit of skepticism. Initially, at least, we often wait to pull these new treatments out of the toolbox for the most severe cases after all other treatments fail to deliver the hoped-for results in our patient’s condition. When these novel treatments don’t pull their unstable ocular condition out of the abyss into which they have fallen, we condemn them as ineffective and not worth the cost.
But when was the last time any of us allowed a patient to maintain a significantly elevated IOP that caused irreversible loss of visual field and retinal nerve fiber layer (RNFL), waiting until the damage progressed to a severe stage before employing a treatment to lower the IOP — and then condemned that medication as ineffective to reverse the RNFL and visual field loss?
In two pivotal trials (one in Europe and the other in the United States) in patients with Mackie classification stage 2 (PED) and stage 3 (stromal ulceration) conditions, more than 70% of patients had completely healed corneas; that is to say, in more than seven out of 10 of these advanced NK presentations there was complete corneal healing with no punctate staining after a single 8-week course of Oxervate therapy. Even more impressive, 80% of these patients remained stable with complete corneal surface healing 48 weeks after completing one 8-week cycle of Oxervate.6,7,11
The safety profile of Oxervate is excellent, with approval for patients as young as 2 years old.7,11 In my experience, the most common complaints are the need to port it around in a thermal container to keep it chilled and the required six-times-a-day application. Application of the drop is a bit different from what we and our patients are used to, but these problems are easily addressed with a little education. When patients note increasing instillation site stinging or burning, often 2 to 3 weeks into the course of their treatment, this can be viewed as a good sign, indicating that corneal sensation and possibly innervation is improving.
Still, a small percentage of individuals, less than 5%, may complain of a deeper orbital pain, often lasting hours and progressing with more prolonged treatment. In these situations, it may be time to discontinue the medication.
CONCLUSION
Addressing and controlling all of the associated ocular surface issues in cases of NK is extremely important. The clinician must identify and treat the additional sources of ocular surface inflammation, such as keratoconjunctivitis sicca, blepharitis, exposure keratitis, allergic conjunctivitis, preservative toxicity and medicamentosa, contact lens-related issues and limbal stem cell deficiency as well as optimizing meibomian gland function. All remain critical to the long-term health of the ocular surface. Regardless of the treatment regimen for NK, long-term treatment to address and control all of these other factors is essential. OM
REFERENCES
- Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018; 66:107-131.
- Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571-579.
- Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the pathogenesis of neurotrophic keraitis: The role of corneal nerves. J Cell Physiol. 2017;232:717-724.
- Chang BH, Groos EB. Neurotrophic Keratitis. In: Mannis MJ, and Holland EJ. Cornea, Vol. 1, 4th edition. London, Elsevier. 2017;1035-1042.
- Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg. 2009;123:112-120.
- Koaik M, Baig K. Corneal neurotization. Curr Opin Ophthalmol. 2019;30;292-298.
- Bonini S, Lambiase A, Rama P, et al. Phase I trial of recombinant human nerve growth factor for NK. Ophthalmology. 2018;125;1468-1471.
- Bonini S, Lambiase A, Rama P, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for NK. Ophthalmology. 2018;125;1332-1343.
- Voelker R. New drug treats rare, debilitating neurotrophic keratitis. JAMA. 2018; 320:1309.
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003; 76:521-542.
- Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (Cenegermin) for neurotrophic keratopathy: A multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127:14-26.









