According to a survey of cataract surgery patients by Tester et al, dysphotopsia is a leading cause of dissatisfaction after cataract surgery (49% of patients).1 Similarly Bournas et al found that dysphotopsia was present in 19% of surgical patients at postoperative day 1.2 Unfortunately, dysphotopsia management can be extremely challenging for both patients and surgeons as this diagnosis carries great morbidity.
Pseudophakic dysphotopsia is defined as unwanted optical images after uncomplicated cataract surgery with a posterior chamber IOL (PCIOL) fully in the capsule bag. The term “dysphotopsia” was first coined by Randall Olsen, MD.
Pseudophakic dysphotopsia can be classified into three categories:
- Positive dysphotopsia (PD). Defined as light streaks, light arcs, halos and starbursts typically stimulated by an oblique light source
- Negative dysphotopsia (ND). Defined as a temporal dark shadow or arc
- Multifocal/diffractive dysphotopsia. Characterized by starbursts, glare and halos with a central point source of light.
Patients can have one or all photic symptoms. This summary article will provide a systematic approach to managing PD and ND — multifocal/diffractive dysphotopsia is better understood and successfully treated by IOL exchange for a monofocal IOL.
Prior to diagnosing pseudophakic dysphotopsia, it is essential to rule out neurological, retinal, iris or capsular pathology. What is most frustrating about this “perfect surgery” complication is the difficulty predicting which patients are predisposed to developing pseudophakic dysphotopsia. Fortunately, medical and surgical interventions can reduce symptoms in affected patients.
In this article, we will focus on the medical and surgical management of this unique and often neglected patient population.
Positive dysphotopsia
Background and etiology
The etiology of PD is best understood of all the dysphotopsiae and is predominantly caused by internal reflection from square-edge design often stimulated by temporal or oblique light. Incidence is somewhere between 19-49% in survey reports.1,2
Truncated or square-edge design of ovoid PCIOLs was first reported as a source of undesired optical imagery by Masket et al.3 Ray-tracing modeling by Holladay et al and Franchini et al described how square-edge IOL design can result in more internal reflection than round-edge IOL designs.4,5 In addition, index of refraction, reflectivity and optic size have been implicated as causative.2-9 This observation led to the assumption that round-edge designs could potentially decrease PD symptoms. However, research by Nishi et al revealed the need for the square posterior edge to impede lens epithelial migration and posterior subcapsular opacification (PCO).10
It is important to distinguish between PD findings and light flashes from retinal vitreous traction or a linear streak from striae in the posterior capsule causing a Maddox rod effect (Figure 1).11 Other posterior capsulotomy findings can be confused with PD, such as a small or asymmetric posterior capsule opening. “Fluttering” is a common complaint in the initial postoperative period and typically resolves quickly after surgery as the capsule fibroses around the IOL.
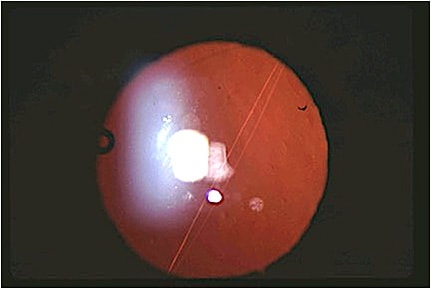
Lastly, corneal aberrations caused by laser refractive surgery or ocular surface disease may complicate the diagnosis. Performing a contact lens trial and/or wavefront aberrometry testing can help to separate the cornea from the lens, confirming the IOL as the inciting cause.
Fortunately, industry has responded to PD issues in the following ways: leaving the edge of the IOL unpolished, increasing the effective optical zone9 or creating a rounded anterior portion of the IOL edge while maintaining a square posterior edge to prevent PCO.5 However, despite the best intentions, a vocal percentage of patients develop persistent PD and need intervention.
PD treatment only
PD is generally present from postoperative day 1 and typically does not improve with time. It is prudent to wait a few months to allow for potential neuroadaptation to the dysphotopic symptoms. However, neuroadaptation to PD is exceedingly rare. During this time, it is helpful to give a miotic agent topically such as pilocarpine 0.5% b.i.d. or brimonidine 0.15% b.i.d. to decrease the pupil size and improve symptoms. If the PD symptoms persist longer than 3 to 6 months, one can consider an IOL exchange for a different lens material and/or edge design and recommend a lower index of refraction. Resist the urge to laser the posterior capsule as this may complicate the IOL exchange.
The ideal treatment for PD involves exchanging the inciting IOL for a round edge,3-7 lower index of refraction IOL6 with a larger optic.9 A lower incidence has also been reported with round-edge and polymethylmethacrylate (PMMA) designs.7 However, as mentioned, we do not have a truly round-edge design on the market in the United States, and PMMA IOLs are not foldable and require a large incision. Two IOLs have a round anterior edge and square posterior edge IOLs that may help decrease PD: the Sensar acrylic 3-piece IOL (J&J Vision) and the copolymer/collamer CQ2015A (Staar Surgical).2
Masket et al (JCRS in editorial review) found that changing the IOL to a lower index of refraction improves symptoms in 76% to 88% of patients. When an acrylic IOL is exchanged for either a silicone or copolymer/collamer PD symptoms were improved. PD symptoms improvement after IOL exchange from acrylic to silicone (LI61AO, Bausch + Lomb) was 87% and from acrylic to copolymer/collamer (CQ2015A, Staar Surgical) was 88%. The difference between groups were not statistically significant. Unfortunately, the CQ2015A is no longer manufactured.
Acrylic IOLs continue to dominate the U.S. market. Therefore, our current strategy is to exchange the acrylic IOL for an L161AO, as it has a lower index of refraction and has been shown to decrease PD symptoms. The caveat is its square optic edge design, so it may not resolve all PD symptoms completely.
Negative dysphotopsia (ND)
Background and etiology
ND is described by patients as a dark temporal peripheral shadow, arc or line. Some patients also describe a ring around their vision resembling “looking through binoculars.” ND can be subcategorized into temporary ND that typically resolves within the first postoperative month and persistent ND that is defined as symptoms lasting greater that 3-6 months.
When a patient presents with ND, it is critical to rule out any retinal or neurological reason for the temporal scotoma with a careful dilated fundus exam and a 30-2 visual field test. Once other pathology is ruled out, a few pearls are helpful in making the diagnosis:
- ND occurs with anatomically “perfect surgery” of any in-the-bag IOL
- Symptoms are exacerbated with a temporal oblique light source
- ND improves with dilation
- ND has been reported with any IOL type or material.8,12
After diagnosis, the next step is to reassure the patient and explain that symptoms of ND improve in 97% of cases over time.13
Davidson reviewed patients with acrylic IOLs and helped clarify the distinction between ND and PD. He found an incidence of persistent ND in 0.2% of patients and confirmed a lower incidence of PD with round-edge IOL design.7 Alternatively, Osher et al found in a prospective study a 15.2% incidence of temporary ND at postoperative day 1 with persistent ND decreasing to 3.2 and 2.4% at 1 and 2 years, respectively.13 It remains challenging to determine the true incidence of ND as not every cataract patient is screened and there are no easily available objective measures. Recently, Goldmann kinetic visual fields have proven useful for mapping ND scotomas.14,15
Ray tracing modeling has evolved to provide us with a working theory of the etiology of ND.16-19 Ray-tracing optical modelling describes the cause of ND as an “illumination gap” due to a narrow band of non-illuminated nasal retina that is bounded posteriorly by light refracted by the optic as intended and bounded anteriorly by light that misses the optic.16-19 It is hypothesized that the ND shadow would be eliminated by illuminating the dark band of nasal retina or by shifting the dark region anteriorly beyond functional retina.16-19 These reports offer a meaningful suggestion for the focal ocular mechanism for ND.
ND treatment only
For persistent ND, the strategies include secondary reverse optic capture (ROC) (Figure 2), sulcus placement, piggyback IOL, Nd:YAG or surgical nasal capsulectomy and, most recently, neuroadaptive therapy. Masket et al reported success rates with various strategies in 61 patients and found 96% improved with ROC, 86% improved with sulcus placement and 73% improved with piggyback IOL.12 No patients improved with in-the-bag IOL exchange alone.

Folden and Cooke described Nd:YAG nasal capsulectomy with a small sample size and a 60% to 80% success rate.20,21 Both Vamosi et al and Burke et al described success with sulcus placement irrespective of high index of refraction IOL.22,23 The working theory of moving the “missing” rays anteriorly fits current surgical strategies to treat ND except for nasal capsulectomy. With both ROC and sulcus placement, the optic is moved forward, potentially moving the “missing rays” anteriorly, avoiding the anterior retina and falling on the visually insensitive pars plana. In a piggyback IOL, the light is scattered by an anterior optic.19,24 Caution is advised with a piggyback IOL, as iris chafing is common and may lead to pigment dispersion and glare.
The challenge with this patient cohort is the difficultly of predicting who will develop ND. Recently, large angle kappa, high IOL power, index of refraction, aphericity, nasal capsule has been implicated and may give us predictive models to counsel patients preoperatively.17,25 If a patient has persistent ND (more than 3 to 6 months) in the first eye, the strategy for the second eye can involve a primary reverse optic capture positioning or sulcus placement, both with a three-piece IOL.12,22 Preventative measures have been described by Henderson et al in which haptics placed horizontally resulted in a significant decrease in the incidence of temporary ND at 1 week. However, by 1 month this effect was no longer statistically significant.26,27
ND and PD treatment
Olsen and others have suggested that a temporal persistent “shimmering or flickering” effect, reported by some patients, may be manifestation of ND and not just PD. This is important because ND and PD can coexist and the treatments require slightly different approaches. For PD, we simply want to change the IOL material and/or edge design; for ND, we want to somehow move the IOL forward, particularly in front of the anterior capsule, using ROC or sulcus placement. Proper overlap of the optic in front of the nasal capsule appears to be paramount, as we have had patients recur with symptoms when this was not adequate.28
Neuroadaptive therapy/CNS implication
Recent pilot investigations suggest that ND has a CNS component and neuroadaptive therapy may be offered prior to surgical intervention. Masket et al reported that the ND scotoma, as measured by automated kinetic Goldmann visual field studies, is reduced in 80% of cases when the contralateral eye is occluded, indicating that ND has CNS manifestations beyond a local retinal illumination gap theory.29 Therefore, ND must continue to be considered as a complex and enigmatic condition, unlike PD, which seems far easier to analyze.
Conclusion
Pseudophakic dysphotopsia remains a frustrating complication of “perfect” cataract surgery for both the patient and surgeon. Fortunately, innovative IOL designs are being studied to address dysphotopsia either by changing edge design curvature to redirect light rays30 or capturing the capsulotomy edge.31
The goal is to develop a means to predict, diagnose and treat dysphotopsia in a systematic and empathetic manner. The medical, neuroadaptive and surgical strategies discussed may improve treatment protocols and allow for targeted treatment to decrease the confusion surrounding pseudophakic dysphotopsia management. OM
References
- Tester R, Pace NL, Samore M, Olson RJ. Dysphotopsia in phakic and pseudophakic patients: incidence and relation to the IOL type. J Cataract Refract Surg. 2000;26:810-816.
- Bournas P, Drazinos S, Kanellas D, Arvanitis M, Vaikoussis E. Dysphotopsia after cataract surgery: comparison of four different intraocular lenses. Ophthalmologica. 2007;221:378-83.
- Masket S, Geraghty E, Crandall AS, Davison JA, Johnson SH, Koch DD, Lane SS. Undesired light images associated with ovoid intraocular lenses. J Cataract Refract Surg. 1993; 19:690-694.
- Holladay JT, Lang A, Portney V. Analysis of edge glare phenomena in intraocular lens edge designs. J Cataract Refract Surg. 1999; 25:748-752.
- Franchini A, Gallarati BZ, Vaccari E. Computerized analysis of the effects of intraocular lens edge design on the quality of vision in pseudophakic patients. J Cataract Refract Surg. 2003;29:342-347.
- Erie JC, Bandhauer MH, McLaren JW. Analysis of postoperative glare and intraocular lens design. J Cataract Refract Surg. 2001;27:614-621.
- Davison JA. Positive and negative dysphotopsias in patients with acrylic intraocular lenses. J Cataract Refract Surg. 2000;26:1346-1355.
- Trattler WB, Whitsett JC, Simone PA. Negative dysphotopsia after intraocular lens implantation irrespective of design and material. J Cataract Refract Surg. 2005;31:841-845.
- Das KK, Werner L, Collins S, Hong X. In vitro and schematic model eye assessment of glare or positive dysphotopsia-type photic phenomena: Comparison of a new material IOL to other monofocal IOLs. J Cataract Refract Surg. 2019;45:219-227.
- Nishi O. Posterior capsule opacification. Part 1: Experimental investigations. J Cataract Refract Surg. 1999;25:106-117.
- Holladay JT, Bishop JE, Lewis JW. Diagnosis and treatment of mysterious light streaks seen by patients following extracapsular cataract extraction. Am Intra-Ocular Implant Soc J. 1985;11:21-23.
- Masket S, Fram NR, Cho A, Park L, Pham D. Surgical management of negative dysphotopsia. J Cataract Refract Surg. 2018;44:6-16.
- Osher RH. Negative dysphotopsia: long-term study and possible explanation for transient symptoms. J Cataract Refract Surg. 2008;34:1699-1707.
- Makhotkina NY, Berendschot TTJM, Nuijts RMMA. Objective evaluation of negative dysphotopsia with Goldmann kinetic perimetry. J Cataract Refract Surg. 2016; 42:1626-1633.
- Masket S, Rupnik Z, Fram NR. Neuroadaptive changes in negative dysphotopsia during contralateral eye occlusion. J Cataract Refract Surg. 2019; 45:242-243.
- Coroneo MT, Pham T, Kwok LS. Off-axis edge glare in pseudophakic dysphotopsia. J Cataract Refract Surg. 2003;29:1969-1973.
- Holladay JT, Simpson MJ. Negative dysphotopsia: causes and rationale for prevention and treatment. J Cataract Refract Surg. 2017; 43:263-275.
- Simpson MJ. Double image in far peripheral vision of pseudophakic eye as source of negative dysphotopsia. J Opt Soc Am A Opt Image Sci Vis. 2014;31:2642-2649.
- Erie JC, Simpson MJ, Bandhauer MH. Effect of a sulcus-fixated piggyback intraocular lens on negative dysphotopsia: Ray-tracing analysis. J Cataract Refract Surg. 2019;45:443-449.
- Folden DV. Neodymium: YAG laser anterior capsulectomy: surgical option in the management of negative dysphotopsia. J Cataract Refract Surg. 2013;39:1110-1115.
- Cooke DL, Kasko S, Platt LO. Resolution of negative dysphotopsia after laser anterior capsulotomy. J Cataract Refract Surg. 2013; 39:1107-1109.
- Burke TR, Benjamin L. Sulcus-fixated intraocular lens implantation for the management of negative dysphotopsia. J Cataract Refract Surg. 2014; 40:1469-1472.
- Vamosi P, Csakany B, Nemeth J. Intraocular lens exchange in patients with negative dysphotopsia symptoms. J Cataract Refract Surg. 2010;36:418-424.
- Hong X, Liu Y, Karakelle M, Masket S, Fram NR. Ray-tracing optical modeling of negative dysphotopsia. J Biomed Opt. 2011;16:125001.
- Makhotkina NY, Dugrain V, Purchase D, Berendschot TTJM, Nuijts RMMA. Effect of supplementary implantation of a sulcus-fixated intraocular lens in patients with negative dysphotopsia. J Cataract Refract Surg. 2018; 44:209-218.
- Henderson BA, Yi DH, Constantine JB. GenevaII. New preventative approach for negative dysphotopsia. J Cataract Refract Surg 2016;42:1449-1455.
- Erie JC, Simpson MJ, Bandhauer MH. Influence of the intraocular lens optic–haptic junction on illumination of the peripheral retina and negative dysphotopsia. J Cataract Refract Surg. 2019; 45:1335-1339.
- Masket S, Fram N. Pseudophakic negative dysphotopsia: Surgical management and new theory of etiology. J Cataract Refract Surg. 2011;37:1199-1207.
- Masket S, Rupnik Z, Fram N, Vikesland R. Binocular Goldmann visual field testing of negative dysphotopsia. J Cataract Refract Surg. 2020 46:147-148.
- Erie JC, Simpson MJ, Bandhauer MH. A modified intraocular lens design to reduce negative dysphotopsia. J Cataract Refract Surg. 2019;45:1013-1019.
- Masket S, Rorer E, Stark W, et al. Special Report: The American Academy of Ophthalmology Task Force Consensus Statement on Adverse Events with Intraocular Lenses. Ophthalmology. 2017;124:142-144.









