Dry eye disease is one of the most common reasons that patients seek an eye-care practitioner, but it’s often not the only pathology contributing to a patient’s clinical picture. In this article, we present three cases that examine how dry eye can be a comorbidity for other conditions.
CASE 1: DRY EYE & CATARACT
The patient
Symptomatic dry eye can affect quality of life, fluctuations in vision and potential suboptimal outcomes with refractive or cataract surgery. Additionally, 86% of patients with dry eye also have meibomian gland dysfunction (MGD) as a primary or contributary cause.1 In this case, we present a patient with a history of both refractive and cataract surgery and undiagnosed concomitant dry eye disease.
A 67-year-old woman presented to clinic with a history of LASIK OU and subsequent cataract surgery OD performed at an outside facility. She was unhappy with her results despite an uneventful cataract surgery, as she ended up with a myopic refraction rather than the goal of emmetropia. She complained of blurry distance vision and ghosting in the treated eye, along with grittiness, tearing and burning, which she never noticed preoperatively. She expressed concern about reduced visual acuity from a progressively dense cataract in the fellow eye. Given the outcome from the first cataract surgery, she was reluctant to return to the original surgeon.
Examination
Exam demonstrated an uncorrected visual acuity of 20/50 J3 OD and 20/30 J2 OS. The operative eye appeared to be about 1.50 D overcorrected despite a distance target and plano in the left eye. Glare testing reduced the vision off the chart OS. Upon examination, the meibomian glands appeared normal, but diagnostic meibomian gland expression of the lower lids revealed thick, toothpaste-like secretions. Although she did not have a prior diagnosis of dry eye, both eyes had significant punctate epithelial keratopathy (PEK) (Figure 1). Tear osmolarity (TearLab) was 315 mOsms/L OD and 307 mOsms/L OS with a positive InflammaDry (Quidel) MMP-9 reading. The IOL was well centered in the right eye, and the left eye had a 2 to 3+ nuclear cataract. IOP was 17 mm Hg OD and 16 mm Hg OS. The posterior segment appeared unremarkable in both eyes.
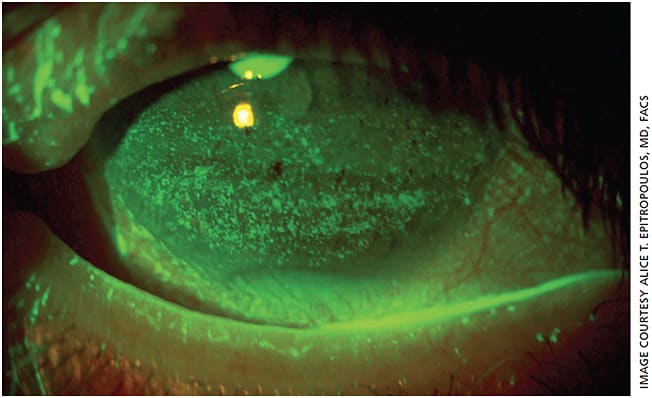
Dry eye disease with a nonobvious MGD component was likely present before cataract surgery in the right eye and likely contributed to inaccurate biometry and topography, leading to the overcorrection. Patients with abnormal tear film osmolarity have significantly more variability in average keratometry (K) readings and anterior corneal astigmatism than those with normal osmolar values.2 The poor-quality tear film and PEK could have further contributed to this patient’s reduced vision after surgery. Before operating on her second eye, we needed to understand how much of her visual complaints were due to the cataract and how much to dry eye. Furthermore, it was important to optimize the health of the ocular surface to obtain accurate biometry and IOL power calculations.
Treatment
We treated the patient with vectored thermal pulsation therapy (LipiFlow, Johnson & Johnson Vision) to address her obstructed meibomian glands and started her on a short course of topical steroids and lifitegrast (Xiidra, Novartis) and re-esterified omega-3 fatty acid supplements (PRN). After initiating these treatments, the patient reported that she already was experiencing less ghosting in the treated eye.
Preoperative biometry was scheduled for 6 weeks after beginning treatment. This delay is essential to ensure biometry is accurate and a proper surgical plan is implemented. Cynthia Matossian, MD, recently presented data showing that eyes with MGD can have significant changes in the magnitude and axis of astigmatism, leading to changes in surgical treatment plans after vectored thermal pulsation therapy.3
The lesson
It is important to take the time to actively screen patients presenting for cataract surgery for pre-existing ocular surface disease, whether they are symptomatic or not. The PHACO study illustrates the prevalence of dry eye in this population. More than 75% had corneal staining, despite a much smaller fraction (22%) having a prior diagnosis of dry eye.4 Similarly, our patient was not aware of having dry eye. In an older-age population, MGD should always be considered. MGD can be nonobvious until one applies mechanical pressure to the lids and/or obtains meibography images.
CASE 2: DRY EYE & EBMD
The patient
Undiagnosed and untreated dry eye can lead to adverse outcomes after refractive or cataract surgery, but sometimes there is more than just one pathology contributing to the clinical picture. In this case, we present a patient interested in cataract surgery with both dry eye disease and epithelial basement membrane dystrophy (EBMD).
A 58-year-old physician was referred for a cataract evaluation. He complained of foreign-body sensation along with intermittent blurring of his vision, affecting his ability to function at work. He expressed an interested in reducing his dependence on spectacles.
Examination
On exam, he had 2+ nuclear and cortical cataracts but was also found to have significant EBMD (Figure 2). While EBMD often affects the periphery, in this case it was affecting the visual axis (central 3.0 to 5.0 mm of the cornea) in his right eye, producing topographic irregularities as can be seen from the distorted Placido ring mires on topography (Figure 3A). At the initial visit, the K readings were 44.23 D and 46.23 D with 2.00 D of cylinder at a 45° axis, and IOL calculations suggested a toric IOL.
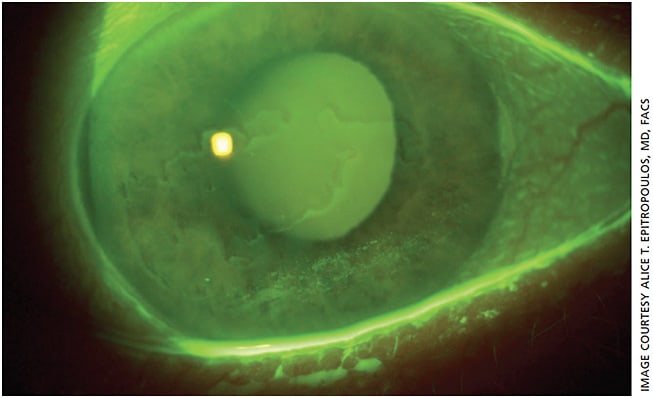
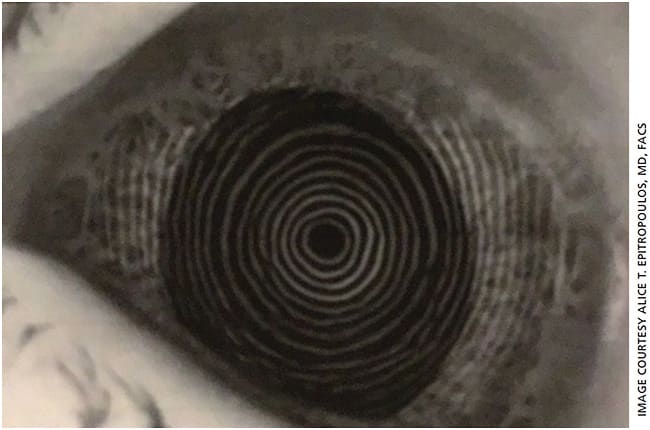
However, irregular topography with distorted central mires is a clear indication that treatment is needed before proceeding with final preoperative measurements and cataract surgery.
Treatment
We started the patient on a topical steroid and immunomodulator, preservative-free artificial tears and re-esterified omega-3 fatty acid supplements to address the dry eye. In addition, the patient underwent an epithelial debridement and phototherapeutic keratectomy (PTK) to treat the EBMD followed by application of an amniotic membrane. Together, these treatments significantly improved the corneal irregularity, providing topography mires that were much less distorted (Figure 3B). The K readings were 45.92 D and 46.49 D with a reduction in cylinder (pseudocylinder) to 0.57 D at 94°, which made a 1.00-D difference in the spherical power. A toric lens was no longer recommended.
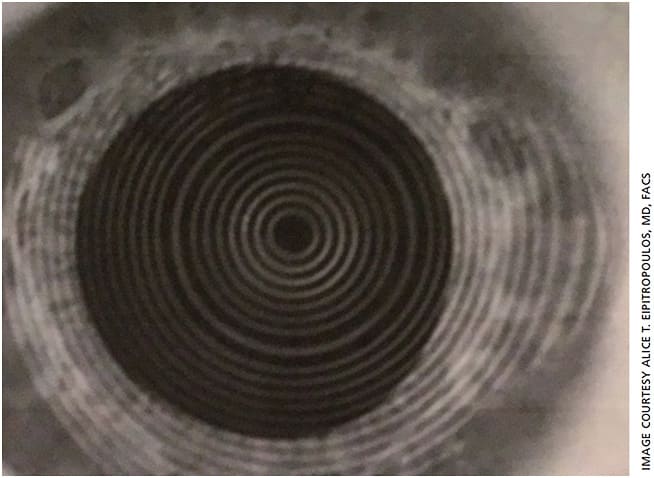
The lesson
Although EBMD may have been the most dramatic comorbidity for this patient, it was important not to minimize the dry eye component. A healthy tear film is needed to promote optimal healing after PTK and cataract surgery. The treatment for EBMD and DED changed our surgical treatment plan, as no toric IOL would be needed and the IOL spherical power changed by a full diopter.
Had we proceeded with surgery based on the initial measurements, this would have led to a suboptimal result. Instead, he is back at work, treating patients in his practice and extremely happy with his vision.
CASE 3: DRY EYE & GLAUCOMA
The patient
While it is known that a certain subset of glaucoma patients can have an allergic or toxic reaction to the preservative in many of the topical drops used to treat increased IOP, topical drops can also contribute to MGD and DED and may be a factor in reduced vision.
In this case, a 38-year-old female patient with primary open-angle glaucoma, controlled on topical dorzolamide, timolol and latanoprost, presented to clinic with red eye, irritation and blurry vision for the past 6 months. She had a history of cataract surgery performed in both eyes in 2012 and admitted to not being fully compliant with her glaucoma drops because she felt they were causing her blurry vision and irritation. She had tried over-the-counter artificial tears with little success.
Examination
On exam, her IOPs were in the low 20s and she was noted to have moderate cupping of her optic nerves in both eyes (Figure 4). Her anterior segment exam revealed PEK, reduced tear break-up time and a reduced tear meniscus. LipiView dynamic meibomian imaging (Johnson & Johnson Vision) showed significant atrophy of the meibomian glands (Figure 5).
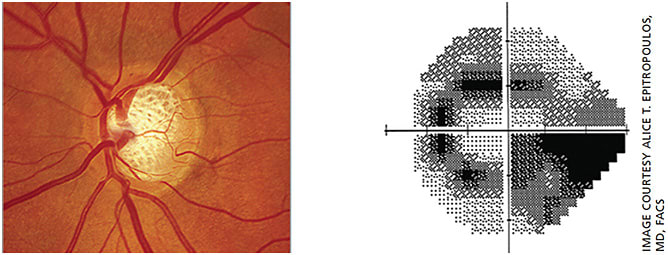
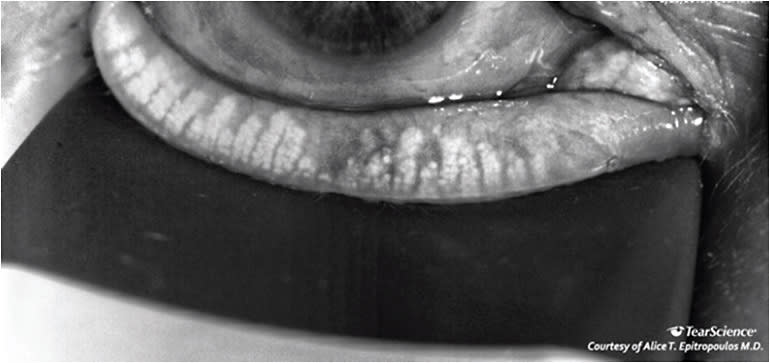
Treatment
The meibography was very helpful in explaining to the patient why she had become so symptomatic. To address her obstructive MGD, we performed vectored thermal pulsation therapy and counseled the patient that repeat treatments may be required in the future. In some instances, one can consider switching the patient from prostaglandin analogs to a different class of glaucoma medications and/or using a preservative-free option.
However, this patient was on two other medications already, leaving us with limited options. We switched her to preservative-free versions of timolol, dorzolamide and tafluprost (Zioptan, Akorn) and prescribed preservative-free artificial tears throughout the day and gel at night.
The lesson
Unfortunately, this is a common presentation for glaucoma patients. Approximately half of glaucoma patients have abnormal Ocular Surface Disease Index (OSDI) scores5 and 60% report mild, moderate or severe symptoms.6 The preservatives in glaucoma drops disrupt tear film stability and ocular surface integrity, so it is of little surprise that the rate of OSD rises with the number of topical medications.7 Moreover, our typical first-line therapeutic drops, prostaglandin analogs, are well-known contributors to MGD.8
Glaucoma patients who have OSD experience far higher rates of side effects from their topical glaucoma therapy than those with no OSD.9 Additionally, patients like the one we presented, with redness, irritation and blurred vision, have a significantly higher risk of glaucoma progression. This may be the case because of poor compliance in response to the unwanted symptoms.
CONCLUSION
Dry eye disease is a common and often undiagnosed pathology that can adversely affect a patient’s quality of life and surgical outcomes. Because it is frequently multifactorial in etiology, a comprehensive history and ophthalmic exam are necessary in order to fully understand how best to manage this treatable condition and its sequelae. OM
REFERENCES
- Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472-478.
- Epitropoulos AT, Matossian C, Berdy GJ, et al. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41:1672-1677.
- Matossian C. The effects of thermal pulsation therapy on astigmatism management in cataract surgery. OSN New York, 2019.
- Trattler WB, Majmudar PA, Donnenfeld ED, et al. The prospective health assessment of cataract patients’ ocular surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423-1430.
- Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618-621.
- Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350-355.
- Rossi GC, Pasinetti GM, Scudeller L, et al. Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur J Ophthalmol. 2013;23:296-302.
- Mocan MC, Uzunosmanoglu E, Kocabeyoglu S, et al. The association of chronic topical prostaglandin analog use with meibomian gland dysfunction. J Glaucoma. 2016;25:770-774.
- Denis P. Adverse effects, adherence and cost-benefits in glaucoma treatment. Eur Ophthalmic Rev. 2011;5:116-122.










