The threat of diabetic retinopathy (DR) is a leading cause of vision loss in developed countries among adults aged 20 to 74 years.1,2 However, unlike other retinal diseases, the vision loss and blindness associated with DR is, in most cases, preventable with appropriate treatment. The advent of anti-VEGF agents, including ranibizumab (Lucentis, Genentech), aflibercept (Eylea, Regeneron) and off-label bevacizumab (Avastin, Genentech), revolutionized the treatment of diabetic macular edema (DME) and DR more broadly. Nevertheless, screening and surveillance of the disease in its early stages remains a public-health challenge in many regions of the world.
Therefore, identification and management of afflicted patients early in their disease course is of paramount importance. Studies from several developed countries have reported remarkable success in reducing rates of blindness due to DR through screening programs.3–5 Additionally, important strides have been made through recent developments in imaging technology and expanded therapeutic approaches. This article highlights some of these clinically relevant advances.
DR OVERVIEW
A primer
DR manifests as an ischemia-driven retinal microvascular complication in patients with diabetes mellitus (DM), with varying levels of severity. With approximately 374 million people living with DM worldwide, it is estimated that one-third of these patients are afflicted with DR.
Further, about one-third of this population will develop severe nonproliferative DR (NPDR) or proliferative DR (PDR), advanced stages of the disease typically associated with extensive retinal non-perfusion (RNP). This RNP leads to retinal hypoxia, resulting in upregulation of a host of cytokines including VEGF-A, a prime driver in the development of DME and neovascularization.6,7
DR SURVEILLANCE
Advancements in retinal imaging
Expanded imaging capabilities are improving our ability to prognosticate in the context of DR. The landmark Early Treatment Diabetic Retinopathy Study (ETDRS) defined the Diabetic Retinopathy Severity Scale (DRSS), the first validated, quantitative scale of DR that allowed predicting rates of DR progression based on DR severity.8 This scale has proven invaluable to both clinical care delivery and drug development. More recently, additional imaging approaches are providing new insight into how clinicians risk-stratify.
Widefield imaging
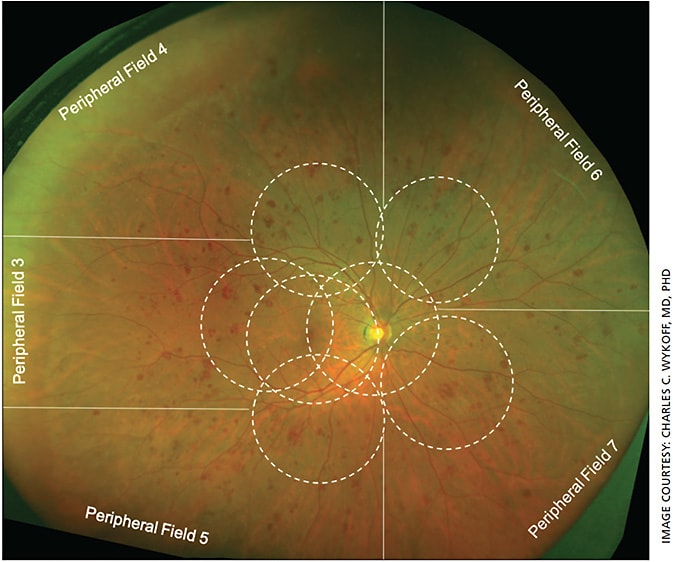
For decades, the 30° seven-field imaging protocol defined by the ETDRS remained the gold standard for DR assessment.9 In the last several years, the introduction of widefield (WF) imaging has transformed DR assessment (Figure 1). Traditional seven-field fundus imaging allows evaluation of approximately 30% of the retinal surface, whereas WF imaging can capture up to 82% of the retinal surface with a single capture,10 providing the opportunity to assess DR lesions outside of the posterior pole (Figure 2).
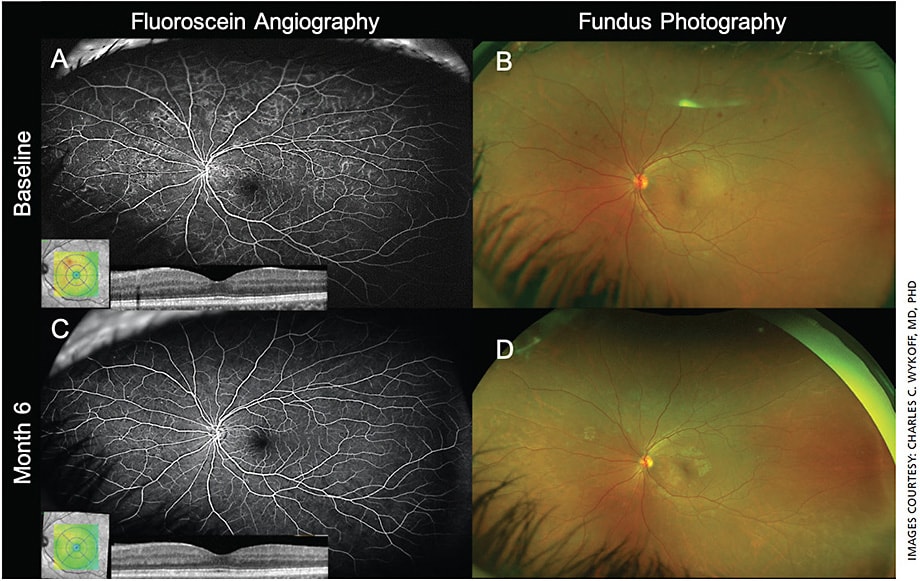
Studies have also demonstrated the agreement and possible superiority of WF photography and fluorescein angiography (FA) in grading DR severity compared to the ETDRS 7-field protocol.11-15
This is an important advance, because predominantly peripheral lesions (PPLs) appear to be a clinically relevant biomarker to inform DR prognostication. As defined by the Diabetic Retinopathy Clinical Research (DRCR) Network study, Protocol AA, a lesion type (hemorrhages/microaneurysms, venous beading, intraretinal microvascular abnormalities or neovascularization elsewhere) is predominantly peripheral in a specific field (Fields 3-7; Figure 2) if >50% of the individual lesions are in the retinal periphery compared with the standard ETDRS fields; an eye is then defined as containing PPLs if any group of graded lesions in any field is predominantly peripheral.12 In one of the initial studies of PPL by Silva et al, approximately one-third of identified lesions were PPLs, located outside of the seven-field range, their findings suggesting a more severe assessment of DR in a clinically meaningful proportion of eyes.11
The presence of PPLs in a substantial proportion of eyes with DR has also been confirmed by a baseline assessment of images within the DRCR Network Protocol AA, with additional data forthcoming from this multi-year prospective study.12 It has been further demonstrated by Silva et al that the presence and extent of PPLs are also associated with increased risk of progression of DR over 4 years.11 For eyes with NPDR at baseline, PPLs in any peripheral field indicated a 3.2-fold increased risk of a two-step worsening of DRSS grade and a 4.7-fold increased risk of developing PDR over 4 years compared to eyes with NPDR and no PPLs. As for eyes with no DR activity on seven-field imaging, eyes with PPLs present at baseline had a 2.5-fold greater risk of developing DR within the posterior pole over 4 years.
RNP and OCTA
In addition to PPLs, the prognostic value of areas of RNP is also being studied. Although historically FA has been the primary imaging modality to identify RNP (and indeed this biomarker was appreciated by the ETDRS as having a meaningful impact on rates of DR progression),15 dye delivery is invasive, time-consuming and risky. More recently, noninvasive methods to identify and quantitate areas of RNP have been developed and refined with optical coherence tomography angiography (OCTA) (Figure 3).16 OCTA takes advantage of high-speed, repetitive, structural OCT imaging to create images that are high-resolution and use motion-contrast to display blood flow through the retina without the need for dye injection.
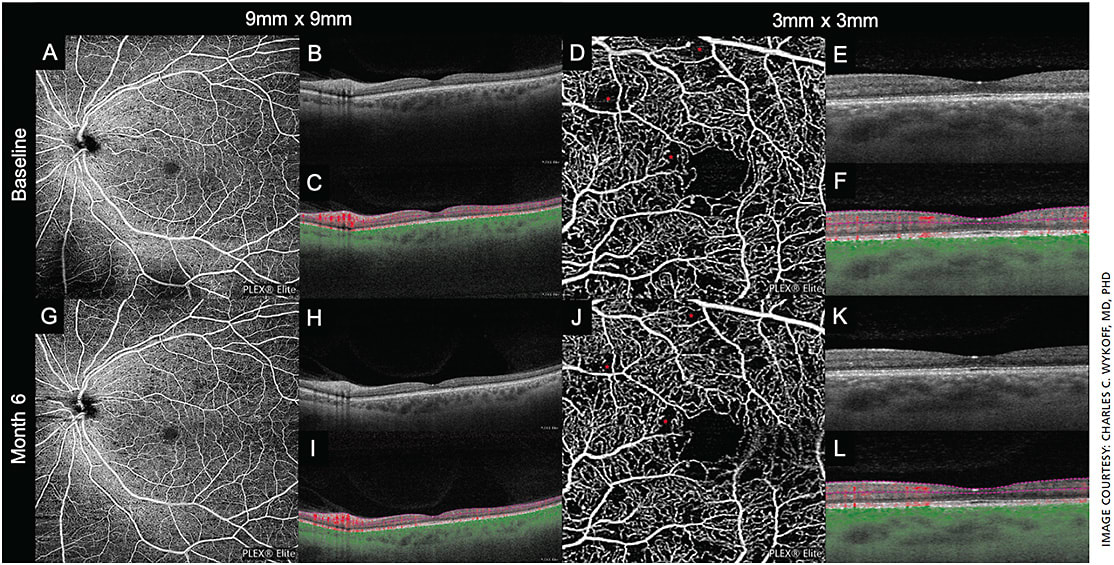
OCTA can also differentiate blood flow between specific retinal layers. This allows for consistent visualization and differentiation of regional blood flow and associated vascular breakdown in the context of DR, a level of resolution not visible with conventional FA or OCT imaging alone.
Several studies have reported the potential application of OCTA for detection of early signs of DR that may precede the visible changes traditionally attributable to DR and furthermore that OCTA can be used to quantitate these changes.16-20 For example, a study of patients without visible DR on fundoscopic examination found subjects to have significantly increased subclinical macular ischemia compared to 40 healthy non-diabetic subjects,21 indicating that OCTA could be used as a screening tool for preclinical DR.
Combining the concepts of WF and OCTA imaging, a 2020 report from the University of Washington highlighted the utility of assessing peripheral RNP with OCTA towards stratifying DR severity.17 Among patients with DM, the OCTA-derived ischemic-index was found to predict DR severity (no DR, NPDR or PDR) with high sensitivity and specificity, with the best predictability reported when more peripheral RNP (between 50° and 100°) was considered. Given the apparent tremendous value of recognizing and quantifying capillary loss in DR,15 it is clear that OCTA, in addition to or possibly instead of more superficial fundus photography, will ultimately prove critically important for classifying DR severity and calculating likely progression rates.19,20
EARLY SCREENING OF PATIENTS WITH DIABETES
We’re not there yet
One of the greatest challenges — and opportunities — in preventing DR-associated blindness is the successful implementation of DR screening among patients with DM. Appropriate and consistent retina screening is critical for identifying DR in its early stages, when it is typically asymptomatic.
Due to effective, population-based DR screening, multiple countries have documented a meaningful decrease in the prevalence of DR-associated visual loss. In the UK, a compelling decrease in the incidence of sight impairment due to DR has been documented.3,4
Also, in 2014, Liew et al reported that, for the first time in at least five decades, DR was no longer the leading cause of certifiable blindness among those aged 16 to 64 years old in England and Wales — attributable to adherence with national DR screening guidelines.3 Similarly, in Poland a meaningful decrease in the incidence rate of blindness between 1989 and 2004 was attributed to the Saint Vincent Declaration of 1989, which set goals for improved diabetic health care, including the introduction of DR screening.5
But, in other developed countries, including the United States, adherence to screening recommendations among patients with DM is woefully suboptimal. Most organizations recommend annual DR-screening for patients with DM.21 A 2019 study reported that nearly one-third of type 1 and half of patients with type 2 DM did not have an eye exam during a 5-year study period, and only 26.3% and 15.3% met diabetic eye disease screening recommendations, respectively.22 Due to a failure of universal screening, DR remains the leading cause of blindness among working-age adults in the United States and is growing in prevalence.1,23
One explanation for these facts and patient loss to follow-up (even once DR is diagnosed)24 is lack of access to ophthalmic care.25 The gold standard for retinal assessment is a dilated eye examination performed by a trained ophthalmologist or optometrist. In addition to this, OCT, fundus photography and FA are often employed in defining extent of DR pathology and directing management decisions. However, for DM patients without access to this level of care, it can be challenging to obtain the recommended DR screening.
AI technology
As a step toward addressing this unmet need, in 2018 the FDA announced its approval for the marketing of the IDx-DR (IDx Technologies), a device designed to detect DR without requiring specialized clinician review.26,27 With this device, fundus photographs are taken with a Topcon camera and uploaded to a server, where an artificial intelligence (AI) program analyzes the images and reports whether the eye is positive for greater than mild DR.
The hope is that this device, and others in development for DR-screening, may substantially improve screening rates, as they allow for screening outside of traditional pathways of care delivery. In a prospective observational study, the IDx-DR AI program correctly identified more than mild DR as graded by the Wisconsin Fundus Photograph Reading Center with a sensitivity of 87.2%, specificity of 90.7% and an imageability rate of 96.1%.28
In November 2019, IDx Technologies reported that IDx-DR was being used in more than 20 institutions across the country, including Johns Hopkins.29 As with nearly every device, the IDx-DR carries limitations. Maybe most critically, images must be captured with a specific camera and the system requires a trained technician to operate.
There remains a need for hardware-diagnostic approaches that allow DR screening to be performed without a human assistant; these could theoretically be deployed in locations frequented by most Americans, such as grocery stores, gas stations and pharmacies. Additionally, IDx-DR’s manufacturer recommends not screening patients with history of a host of retinal treatments, including laser, surgery or intraocular injections.26
CONCLUSIONS
Advances in WF and OCTA imaging have improved our ability to detect DR and stratify DR severity. Recent research as well as other ongoing studies will continue to provide important data to help ophthalmologists determine when and how to intervene at earlier stages of DR.
In this context, there remains a need for better, more consistent screening and surveillance of patients with DM to reduce rates of DR-related visual impairment. OM
Treatments for diabetic eye disease
Coupled with widespread and consistent DR screening, early pharmacologic intervention can play an important role in maintaining and improving visual function. First, anti-VEGF therapy can significantly blunt the progression of NPDR to PDR. For example, PDR events were reduced at the 2-year primary endpoint in the RISE/RIDE study program from approximately 34% with sham treatment to approximately 11% with monthly ranibizumab injections.30
Second, anti-VEGF therapy not only slows DR progression, it can improve DR severity levels as assessed by fundus photography in a substantial proportion of eyes. Additionally, through analyses of multiple Phase 3 DME programs, it appears that high-risk NPDR eyes may receive the greatest benefit from anti-VEGF treatment.31 Finally, while anti-VEGF dosing does not appear to lead to wide-spread reperfusion of areas of RNP,32 consistent dosing has been demonstrated to slow the development and worsening of RNP,33,34 the hallmark of DR, suggesting an ability to truly disease-modify longitudinally.
More recently, the role of anti-VEGF therapies among eyes with high-risk NPDR have been evaluated. The recently completed 2-year, Phase 3, double-masked PANORAMA trial (ClinicalTrials.gov : NCT02718326) randomized treatment-naïve eyes with moderately severe to severe NPDR (DRSS levels 47 to 53) without center-involved DME (n=402) to either sham or two dosing regimens of aflibercept.35 In the second year, the every 4-month (Q16) aflibercept arm continued fixed Q16 dosing and maintained the proportion of patients who achieved an improvement of two or more DRSS steps at about 62% with a mean of just 2.6 injections.
In comparison, in the second year the every 2-month (Q8) aflibercept arm transitioned to PRN re-treatment, based on investigator determined DRSS level; using this approach, the proportion of patients who achieved an improvement of two or more DRSS steps decreased from 80% at 1 year to 50% at 2 years, with a mean of 1.8 injections. Of primary clinical relevance, using a Kaplan Meier analysis accounting for discontinued patients, nearly 58% of sham eyes developed either PDR or center-involved DME by the end of year 2. Using either approach of aflibercept dosing, that number was reduced by about 75%, to about 19%.30
Similarly structured, the ongoing DRCR Network Protocol W (ClinicalTrials.gov : NCT02634333) is a 4-year prospective trial in which eyes with DRSS levels 43, 47 or 53 have been randomized to sham injections or 2-mg aflibercept given at baseline, month 1, month 2, month 4 and then every 4 months until the 2-year primary endpoint. The primary outcome is the cumulative probability of six endpoints indicating either the development of PDR or DME necessitating treatment. Results from 2-year outcomes may be available as soon as late 2020.
Treatment of DR is an actively evolving space, with more ophthalmologists apparently considering interventional therapy for earlier stages of DR. According to the 2019 Preferences and Trends (PAT) survey from the American Society of Retina Specialists, 19.3% of U.S. physicians surveyed indicated that within the previous year, their use of anti-VEGF for eyes with NPDR and no DME had increased; 39.3% also indicated that results from the PANORAMA trial have encouraged them to more frequently initiate early treatment of NPDR without DME and/or increase frequency of visits for these patients.36 This ongoing evolution of practice-patterns in the clinical care of patients with DR will hopefully ultimately impact rates of DR-associated visual impairment and blindness.
References
- Kempen JH, O’Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552-563.
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124-136.
- Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16-64 years), 1999-2000 with 2009-2010. BMJ Open. 2014;4:e004015.
- Thomas RL, Luzio SD, North RV, et al. Retrospective analysis of newly recorded certifications of visual impairment due to diabetic retinopathy in Wales during 2007–2015. BMJ Open. 2017;7:e015024.
- Bandurska-Stankiewicz E, Wiatr D. Diabetic blindness significantly reduced in the Warmia and Mazury region of Poland: Saint Vincent Declaration targets achieved. Eur J Ophthalmol. 2006;16:722-727.
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843.
- Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17.
- Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:823-833.
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786-806.
- Silva PS, Cavallerano JD, Sun JK, et al. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120:2587-2595.
- Silva PS, Cavallerano JD, Haddad NMN, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122:949-956.
- Aiello LP, Odia I, Glassman AR, et al. Comparison of early treatment diabetic retinopathy study standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137:65-73.
- Wessel MM, Aaker GD, Parlitsis G, et al. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32:785-791.
- Ghasemi Falavarjani K, Wang K, Khadamy J, Sadda SR. Ultra-wide-field imaging in diabetic retinopathy; an overview. J Curr Ophthalmol. 2016;28:57-60.
- Fluorescein angiographic risk factors for progression of diabetic retinopathy. ETDRS report number 13. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:834-840.
- Tey KY, Teo K, Tan ACS, et al. Optical coherence tomography angiography in diabetic retinopathy: a review of current applications. Eye Vis. 2019;6:37.
- Wang F, Saraf SS, Zhang Q, Wang RK, Rezaei KA. Ultra-widefield protocol enhances automated classification of diabetic retinopathy severity with OCT angiography. Ophthalmol Retina. 2020;4:415-424.
- Yasin Alibhai A, Moult EM, Shahzad R, et al. Quantifying microvascular changes using OCT angiography in diabetic eyes without clinical evidence of retinopathy. Ophthalmol Retina. 2018;2:418-427.
- Thompson IA, Durrani AK, Patel S. Optical coherence tomography angiography characteristics in diabetic patients without clinical diabetic retinopathy. Eye (Lond). 2019;33:648-652.
- Rosen RB, Andrade Romo JS, Krawitz BD, et al. Earliest evidence of preclinical diabetic retinopathy revealed using optical coherence tomography angiography perfused capillary density. Am J Ophthalmol. 2019;203:103-115.
- Diabetic Retinopathy PPP 2019. American Academy of Ophthalmology. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp . Accessed April 1, 2020.
- Benoit SR, Swenor B, Geiss LS, Gregg EW, Saaddine JB. Eye care utilization among insured people with diabetes in the U.S., 2010-2014. Diabetes Care. 2019;42:427-433.
- Ko F, Vitale S, Chou C-F, et al. Prevalence of nonrefractive visual impairment in US adults and associated risk factors, 1999-2002 and 2005-2008. JAMA. 2012;308:2361-2368.
- Suresh R, Yu H, Thoveson A, et al. Loss to follow-up among patients with proliferative diabetic retinopathy in clinical practice. Am J Ophthalmol. 2020:S0002-9394(20)30111-30112.
- Fathy C, Patel S, Sternberg P, Kohanim S. Disparities in adherence to screening guidelines for diabetic retinopathy in the United States: a comprehensive review and guide for future directions. Semin Ophthalmol. 2016;31:364-377.
- FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye . Published April 11, 2018. Accessed April 1, 2020.
- Savoy M. IDx-DR for Diabetic Retinopathy Screening. Am Fam Physician. 2020;101:307-308.
- Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. Npj Digit Med. 2018;1:39.
- Carfagno J. IDx-DR, the First FDA-Approved AI System, is Growing Rapidly. DocWire News. https://www.docwirenews.com/docwire-pick/future-of-medicine-picks/idx-dr-the-first-fda-approved-ai-system-is-growing-rapidly/ . Published November 12, 2019. Accessed February 14, 2020.
- Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013-2022.
- Wykoff CC, Eichenbaum DA, Roth DB, et al. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmol Retina. 2018;2:997-1009.
- Wykoff CC, Nittala MG, Zhou B, et al. Intravitreal aflibercept for retinal nonperfusion in proliferative ciabetic retinopathy: outcomes from the randomized RECOVERY trial. Ophthalmol Retina. 2019;3:1076-1086.
- Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121:1783-1789.
- Wykoff CC, Shah C, Dhoot D, et al. Longitudinal retinal perfusion status in eyes with diabetic macular edema receiving intravitreal aflibercept or laser in VISTA study. Ophthalmology. 2019;126:1171-1180.
- Wykoff C. PANORAMA: A Phase 3, Double-Masked, Randomized Study of The Efficacy and Safety of Aflibercept In Patients with Moderately Severe to Severe NPDR. Week 100 Results. Presented at the: Angiogenesis; Feb, 8, 2020; Mandarin Oriental Hotel, Miami, Fla.
- American Society of Retina Specialists. 2019 Membership Survey Preferences and Trends. Availble only to ASRS members.










