MDbackline assists by automating patient communication, data collection.
In a busy practice, dry eye patients represent both an opportunity and a challenge. The opportunity to provide better, life-enhancing care is enticing. However, taking the time necessary to understand these patients and educate them on available treatment options can put both doctors and staff behind schedule. At Cataract & Refractive Center of Ohio, we recently implemented MDbackline, a system that automates patient communication and history collection, and it has been a game-changer for our dry eye procedure program.
CONTACTING PATIENTS
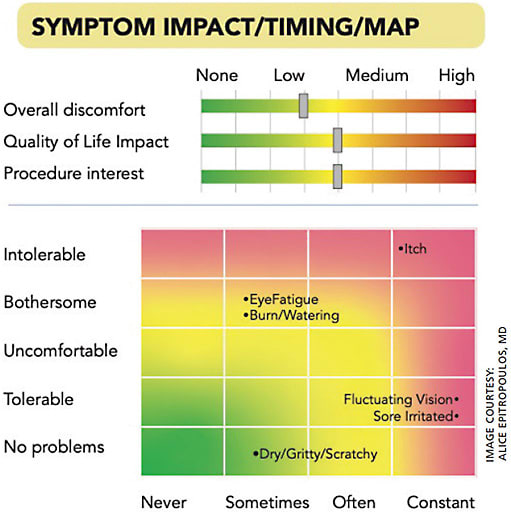
MDbackline (mdbackline.com ) software is an automated, online system that interfaces with our practice management system, identifying patients with incoming appointments for dry eye. Patients are contacted by text message and email to invite them to answer questions about their ocular history and offer them educational material about dry eye. The responses to these questions provide our practice with a one-page “Dry Eye Visual Profile Report” that graphically shows the severity and impact of the patient’s symptoms. It includes medication and medical history, which helps to identify contributors to dry eye as well as what treatments have been used in the past. By looking at the report for just a few seconds, we can identify likely next steps for the patient.

TREATMENTS
The system also reports on the patient’s likelihood of choosing a dry eye procedure based on a variety of responses to questions about symptom impact, the number of previous treatments and their past treatment costs (for more on dry eye treatments, see page 14). This allows us to present advanced treatment options preferentially to those patients for whom they are best suited. Furthermore, the conversation is shorter because those patients are already pre-educated by MDbackline on these treatment options.
We frequently find that patients who don’t spontaneously report their symptoms have surprisingly high degrees of impairment from dry eye, manifested as fluctuating vision or other symptoms. Without effort by humans, those symptoms are elicited and highlighted by the dry eye module. As it turns out, many of these same patients, who are at earlier stages of disease, are the best candidates for advanced treatments, even though they don’t always report their symptoms without being asked.
FOLLOW-UP
After these patients complete their visit to the office, MDbackline follows up with them again with a simple questionnaire to ask if they are satisified with their post-visit treatment. This is perfect for identifying patients who chose to go with conservative treatment, such as lubricating drops and warm compresses, at first but ultimately need more advanced therapy. Also, since many patients are very satisfied with treatment, it refers those patients to online review sites so they can tell others about the care they received.
CONCLUSION
For our practice, MDbackline has transformed dry eye treatment, making our intake process more efficient, our histories more accurate and our use of advanced therapies more widely accepted. We have also derived great value from MDbackline’s patient history and education for cataract patients that is driving adoption of premium lens implants.
Simple solutions like MDbackline allow me to spend more quality time with my patients, building relationships and streamlining routine communication and education. We become better doctors when we have more touch points with our patients, giving us more ways to make their lives better. OM
QUICK NOTES
Ocular Therapeutix was granted a permanent Category I CPT procedure code to replace the currently available Category III CPT code (0356T) for the administration of drug-eluting intracanalicular inserts, including DEXTENZA (dexamethasone ophthalmic insert) 0.4 mg. The code will be effective Jan. 1, 2022.
Bausch + Lomb released the SimplifEYE IOL delivery system, making it the first toric preloaded IOL in the United States. The SimplifEYE is available exclusively for the enVista MX60PL and enVista toric MX60PT platforms. The system’s SnapSet cartridge houses the IOL, and the lens can be placed into the capsular bag through an incision as small as 2.2 mm.
Kala Pharmaceuticals announced the FDA approval of EYSUVIS for the short-term treatment of the signs and symptoms of dry eye disease. EYSUVIS (loteprednol etabonate ophthalmic suspension) 0.25% uses Kala’s AMPPLIFY mucus-penetrating particle technology to enhance penetration of loteprednol etabonate into target tissue on the ocular surface.
Allergan announced positive Phase 3 results for its clinical trials GEMINI 1 and GEMINI 2. The trials evaluated the efficacy, safety and tolerability of investigational AGN-190584 (pilocarpine 1.25%) ophthalmic solution for the treatment of symptoms associated with presbyopia. The trials met their primary efficacy endpoints.
Norlase received FDA approval for its new LION laser system. The LION is a green laser photocoagulator fully ntegrated into a Keeler indirect ophthalmoscope. It is battery powered and utilizes an advanced wireless interface with voice control of parameters; it does not use a fiber tether.








