Preexisting dry eye disease (DED) is extremely common in patients undergoing refractive cataract surgery, and it is a condition that is often underdiagnosed. The PHACO study demonstrated close to 80% of patients scheduled for cataract surgery had evidence of DED and only a small percentage (22%) had a previous diagnosis of this disease.1
In addition, DED is one of the more common reasons why some patients are dissatisfied with their results postoperatively. It is well established that ocular surface disease can cause unpredictable biometry and IOL calculations.2
There are three major reasons why I feel patients should be screened and aggressively treated for dry eye before refractive cataract surgery:
- The tear film is the most important refracting surface of the eye, so an unstable or unhealthy tear film can result in inaccurate biometry and keratometry and suboptimal refractive outcomes postoperatively. This is crucial for patients receiving premium IOLs. They expect excellent outcomes, which an unhealthy ocular surface can prevent.
- Patients with preexisting DED should be educated that symptoms are likely to become more noticeable postoperatively even if they are asymptomatic preoperatively. They should know the reason is their disease, not surgery.
- Treating lid disease and blepharitis and eliminating bacterial overgrowth helps to reduce the dreaded risk of infection or endophthalmitis.
SCREENING
First steps
A great first step to screening for dry eye is the use of a questionnaire, such as the Standardized Patient Evaluation of Eye Dryness, or SPEED, questionnaire and Ocular Surface Disease Index (OSDI), to assess patient perception of ocular surface problems. Based on the patient response, further testing may be indicated. Nevertheless, considering the high percentage of asymptomatic dry eye patients, consider assessing all surgical patients for signs and symptoms of ocular surface disease (OSD). Current options for evaluating and diagnosing DED have improved dramatically over the last several years with point-of-care testing.
Osmolarity and MMP-9
Analyzing the tear film using tear osmolarity, for example, can help diagnose and evaluate response to treatment over time. Tear osmolarity is considered abnormal when greater than 308 mOsmol/L or when there is a difference greater than 8 mOsmol/L between the eyes. In the Journal of Cataract and Refractive Surgery, my co-authors and I demonstrated that hyperosmolar patients had greater variability in keratometry readings and IOL power calculations when compared to patients with normal osmolarity.2
Another test commonly used to guide treatment decisions is the matrix metalloproteinase-9 (MMP-9) to assess for inflammation in the tear film. Levels of 40 ng/mL or higher are considered abnormal.
Meibomian gland dysfunction
Meibomian gland dysfunction (MGD) is thought to be the leading cause of DED (more than 85% of dry eye patients have MGD).3
MGD results in tear film irregularities and subsequent inaccurate preoperative K readings and IOL power calculations. Because MGD can be present without symptoms or visible signs (non-obvious MGD), it’s imperative to evaluate lids, lid margins and meibomian glands. The ASCRS dry eye algorithm focuses on preparing patients for cataract surgery and recommends “look, lift, pull and push,” or LLPP, to comprehensively evaluate for OSD in our preoperative patients.4
Meibography has become an important screening tool. Meibography allows us to identify patients with MGD early, before irreversible damage to the glands, and it’s also a great opportunity to educate our patients and guide the discussion about the disease (Figure).
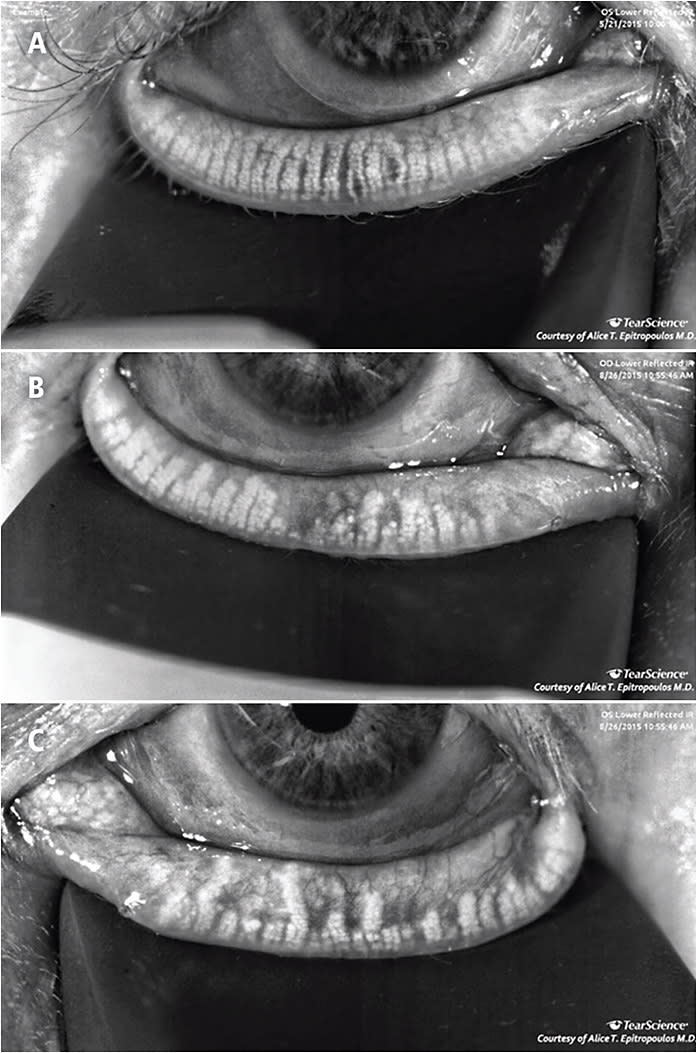
Other diagnostic tools
Topography is another valuable tool that we should use for our preoperative cataract evaluations. Pathologies such as epithelial basement membrane dystrophy, irregular astigmatism, keratoconus and DED that may not otherwise be seen on exams can be identified using topography.
It is also important to establish if patients considering a presbyopic-correcting IOL or LASIK have an ocular surface healthy enough to withstand the challenge of surgery. Sometimes it is difficult to determine whether a patient’s visual complaints are due to cataracts or dry eye and the relative contributions of each. A number of clinical tools have been developed for the assessment of wavefront aberrations of the eye to help determine the cause of visual complaints. Measuring optical scatter through technologies like the HD Analyzer (Visiometrics), iTrace (Tracey) or OPD-Scan III (Nidek) can be helpful in determining which part of the eye is causing the visual distortions that the patient is seeing.
Despite all the point-of-care tests we have available, the hallmark for an accurate diagnosis remains a good history and careful clinical exam. Traditional diagnostics such as TBUT, evaluating the lids and lid margins, quality of tear film and corneal/conjunctival staining can help distinguish between aqueous deficient and evaporative DED, and should be incorporated in every patient exam.
TREATMENT
After the DED diagnosis
Our goal in treating this condition is reducing inflammation and restoring ocular surface homeostasis. Managing DED should be individualized and based on the type and severity of the disease. We have several dry eye algorithms to help guide its diagnosis and treatment. These algorithms have benefits, but we should not view them as a rigid, stepwise approach. Rather, they are organizational tools to aid our treatment decisions.
Of the several available therapeutic options for managing DED, most have become available within the last decade. As new research data emerge, more treatments will continue to be introduced into the armamentarium as innovative products develop and receive approval.
Therapeutic options for MGD
Patients with evidence of obstruction and inflammation of the meibomian glands should consider undergoing a procedure that addresses this dysfunction. This may include LipiFlow thermal pulsation (Johnson & Johnson Vision), iLux (Alcon) or intense pulse light therapy (IPL), although IPL is not approved for DED/MGD. My preference is performing thermal pulsation, as it heats the lids and evacuates the old stagnant meibum and is very effective in relieving gland obstruction and restoring function. I perform LipiFlow in combination with BlephEx to remove the biofilm and bacterial load on the lids and lashes. Cynthia Matossian, MD, presented her data at last year’s ASCRS meeting, which showed that thermal pulsation treatment prior to cataract surgery improves the accuracy of keratometry and subsequent surgical decision making.5
After addressing the obstruction, traditional treatments such as lid hygiene and warm compresses seem to be more effective and help maintain the benefit of the initial thermal pulsation therapy. In addition, I prefer a lipid-based, preservative-free lubricant for my patients with MGD or autologous tears in more advance cases.
Supplements
Although controversial, omega-3 fatty acid supplementation for DED has gained wide acceptance as a primary therapy for dry eye and has become the subject of several clinical investigations. We published our data on the benefits of taking a high quality re-esterified omega-3 supplement (PRN), which demonstrated statistically significant improvement in tear osmolarity, OSDI symptom scores, TBUT, MMP-9 and omega-3 index scores.6
In the DREAM study, patients in both omega-3 and olive oil placebo groups experienced significant improvement in OSDI symptom scores (but the difference between the two groups was not statistically significant).7 A subsequent meta-analysis evaluating 17 peer-review publications showed significant improvements in signs and symptoms of DED with omega-3 compared to placebo.8
Controlling inflammation
Immunomodulators (cyclosporine 0.05% [Restasis, Allergan], cyclosporine 0.09% [Cequa, Sun Ophthalmics], cyclosporine 0.1% [Klarity, Imprimis] or lifitegrast [Xiidra, Novartis]), possibly in conjunction with a short course of a topical steroid, should be considered when patients have signs of inflammation. Punctal plugs are also an option once the inflammation is addressed. These treatments have to be balanced with reduction of the BAK burden from topical drops, including glaucoma medications and artificial tears.
Other options
Risk factors for DED are ubiquitous. Prolonged digital device use can exacerbate DED, so we recommend frequent breaks, blinking exercises and lubricating drops. Drafts and wind also tend to worsen dry eye symptoms, so controlling environmental variables, such as avoiding ceiling fans, can make a difference in the health of the surface.
Coupling various therapies can be effective, especially in more severe cases. If a patient has severe corneal staining, I use a PROKERA amniotic membrane (Bio-Tissue), which has growth factors and nutrients to help enhance and speed healing, along with an immunomodulator, a good quality omega-3 supplement and preservative-free artificial tears and treating obstructive MGD.
Patients that have corneal “stain without pain” should be evaluated for neurotrophic keratitis (NK). Reduced or absent corneal sensation is a hallmark of NK and should be tested when this condition is suspected, because it can be progressive and lead to permanent damage. If NK is confirmed, cenegermin (Oxervate, Dompe) can be a consideration.
Continuing with surgery
Bring back patients for repeat biometry 4 to 6 weeks after dry eye treatment is initiated. If measurements are reliable and good quality, then we can proceed with cataract surgery. In some circumstances, patients return for final biometry after being treated for DED and the vision improves enough that cataract surgery may not be necessary.
If we are unable to optimize the ocular surface and obtain reproducible keratometry and topography, then I am hesitant to recommend a premium IOL. However, most patients who are compliant with dry eye treatment have success with these premium implants. If the surface is still not optimized, though, don’t hesitate to delay surgery until the surface is healthy enough to generate accurate measurements.
DED is extremely common and underdiagnosed in patients presenting for cataract surgery. Screening for DED and optimizing the health of the ocular surface preoperatively is critical to maximizing cataract and refractive surgery outcomes. OM
REFERENCES
- Trattler WB, Majmudar PA, Donnenfeld ED, et al. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423-1430.
- Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41:1672-1677.
- Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea 2012; 31:472–478
- Starr CE, Gupta PK, Farid M et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45:669-684.
- Matossian C. Effect of thermal pulsation system treatment on keratometry measurements prior to cataract surgery. ASCRS 2019.
- Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35:1185-1191.
- Dry Eye Assessment and Management Study Research Group, Asbell PA, Maguire MG, et al. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N Engl J Med 2018. 2018;378:1681-1690.
- Giannaccare G, Pellegrini M, Sebastiani S, et al. Efficacy of omega-3 fatty acid supplementation for treatment of dry eye disease: a meta-analysis of randomized clinical trials. Cornea. 2019;38:565-573.









