Dry eye disease (DED) promises to keep ophthalmologists busy. According to the AAO, it affects approximately 3.2 million women and 1.68 million men over age 50 with likely millions more outside of that age bracket who also suffer from DED, particularly as the use of screens flourishes. Additionally, dry eye in cataract patients is often underreported and underappreciated. The PHACO study of the effect of dry eye in cataract patients found that 77% of eyes scheduled for phacoemulsification had positive corneal staining, and 50% had central corneal staining.1
That great a number of people with DED means that ophthalmologists need a range of diagnostic tools to help pinpoint causes of dry eye and provide tailored treatment. Fortunately, we have a growing number of diagnostic devices — along with long-standing tests — to help evaluate dry eye in our patients. Here is a closer look at the range of dry eye diagnostic tools and tests that are available.
DRY EYE QUESTIONNAIRES
Use of a dry eye-focused, validated psychometric questionnaire is an excellent first step to identify patients affected by dry eye. The Ocular Surface Disease Index (OSDI) questionnaire is one commonly used option, but we have others at our disposal, including the Standard Patient Evaluation of Eye Dryness Questionnaire (SPEED) and Symptom Assessment in Dry Eye (SANDE). These questionnaires help you and your team collect information, and they provide technicians with guidance on whether to proceed with further dry eye testing before you even see the patient.
TEAR OSMOLARITY TESTING
After a psychometric questionnaire, the use of a tear osmolarity test is the next diagnostic tool I choose to have for patients. Hyperosmolarity is the core mechanism by which dry eye syndrome damages the ocular surface. Hyperosmolarity causes inflammation and apoptosis and reduces the ability of mucins to lubricate. It also leads to a breakdown of homeostatic control, causing tear film instability.
The importance of tear film osmolarity is backed by 45 years of peer-reviewed research. Nowadays, hyperosmolarity is considered a marker of dry eye, indicating a concentrated tear film. One study found a positive predictive value of 87% for tear film osmolarity compared with only 33% for the Schirmer test, 33% for tear meniscus height and 25% for tear film breakup time.2
The most well-known tear osmolarity test is made by TearLab. Tear osmolarity results are objective and rapid, and the test is easy to implement and requires a relatively small capital investment. The results aid you and your team with diagnosis and can be repeated to track a patient’s response to therapy.
The TearLab Osmolarity Test is performed with a device that resembles a pen. After patients blink a few times, the “pen” is placed on the inner lid near the corner of the eye until it makes a beeping sound. At that point, one 50-nL tear sample has been collected. Results are available in about 3 minutes.
A result of 300 to 320 mOsm/L indicates mild hyperosmolarity; 320 to 340 mOsm/L is moderate hyperosmolarity; and 340 mOsm/L or higher indicates severe hyperosmolarity. Another indication of hyperosmolarity is an intra-eye difference of 8 mOsm/L or more.
A few pearls to keep in mind when performing tear osmolarity testing:
- Do not let your technicians discuss the results with patients, as tempting as it may be to do so. If asked questions, a simple reply such as, “The doctor will be here in a minute and will explain everything” should keep curiosity at bay. This ensures that the results are explained appropriately.
- Have a tear osmolarity unit in each exam lane. The unit can be used to document dry eye in patients who have dry eye findings at the slit lamp but who do not otherwise have dry eye symptoms (approximately 50% of patients with clinically significant dry eye do not have symptoms, in my experience).
MMP-9 TESTING
The next type of dry eye diagnostic test that I recommend offering is matrix metallopeptidase-9 (MMP-9) testing. I usually use this in conjunction with tear osmolarity testing.
MMPs are proteolytic enzymes produced by stressed epithelial cells on the ocular surface. These enzymes destabilize the tear film and directly contribute to corneal barrier dysfunction by breaking down tight junctions and facilitating inflammatory cell migration. Identifying elevated levels of inflammation and the biomarker MMP-9 can help guide therapeutic decision-making. Down regulation of MMP-9 expression is associated with an improvement in ocular surface epithelial health.3
A normal range for MMP-9 is 3 to 41 ng/ml. Results from MMP-9 testing are a sensitive diagnostic marker and tend to correlate with clinical exam findings.4 Additionally, a higher MMP-9 reading may predict which patients will respond to anti-inflammatory therapy, as not all dry eye patients have clinically significant inflammation. (Traditional dry eye testing methods such as tear film break-up time, Schirmer testing and osmolarity cannot predict which patients have inflammation.) Testing devices for MMP-9 are generally a small capital investment.
InflammaDry (Quidel) is the first and only rapid, CLIA-waived, in-office test that detects MMP-9. InflammaDry accurately detects higher levels of MMP-9 in tear fluid samples taken from the palpebral conjunctiva. It has 85% sensitivity and 94% specificity, according to the company.5
The InflammaDry test is performed by dabbing the sample collector in six to eight locations on the palpebral conjunctiva to collect a tear sample. Red and blue lines indicate positive results, while a blue line (without the red line) indicates negative results. Clinicians typically get the results within 10 minutes. InflammaDry testing can be performed by a technician or nurse.
Make sure to use InflammaDry before using any type of ocular anesthetic, topical dyes or Schirmer testing to ensure an accurate tear sample. Even if a patient has used artificial tear drops, wait an hour before performing the InflammaDry test.
The combined findings from MMP-9 testing and tear osmolarity can help guide your treatment decisions. For example, if a patient has positive results from both the MMP-9 and tear osmolarity testing, then it indicates inflammatory dry eye syndrome. If tear osmolarity is negative and MMP-9 is positive, there may be another cause of inflammation aside from dry eye. Look again for conditions such as allergic conjunctivitis that may have a negative impact on your patient.
Also, keep in mind that osmolarity may fluctuate; while a high osmolarity is an indicator of dry eye, a one time normal osmolarity does not necessarily rule out dry eye. A consistently normal osmolarity over several tests, on the other hand, should trigger consideration of another etiology.
MEIBOGRAPHY
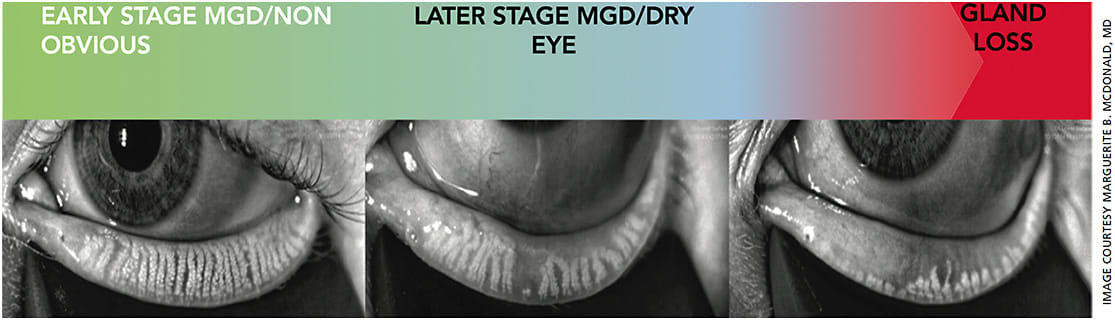
Once you and your staff are comfortable with the diagnostic tests outlined above, it’s time to incorporate meibography. Meibography provides morphologic imaging of the meibomian glands, so it is helpful in diagnosing and grading blepharitis. Meibography is a powerful tool to motivate patients, as they can see the imaging results. It helps them to accept their treatment plan and stay on track to perform both maintenance and preventive care.
Several manufacturers make devices that perform meibography. One such device is the OCULUS Keratograph 5M, which offers several ways to measure the tear film and the health of the ocular surface. The device includes an advanced corneal topographer, a keratometer and a color camera. This technology can be used to perform meibography, measure non-invasive tear film break-up time, assess the thickness of the lipid layer of the tear film, measure tear meniscus height and evaluate the viscosity of the tears. The device also objectively grades conjunctival hyperemia and ciliary flush.
In addition to the OCULUS, other meibography devices are available in the United States, including the LipiView II and LipiScan (Johnson & Johnson Vision) and the Meibox/MX2 (Box Medical Solutions).
When using meibography, look out for duct dilation, gland constipation, tortuosity, atrophy and a hazy appearance (Figure). These findings all indicate that possible treatment is needed.
IMPROVING ON LONG-TIME TESTS
Traditional tests associated with dry eye diagnosis, such as the Schirmer test and tear film break-up time test, are still available options for patients. Patients generally find the Schirmer test uncomfortable, but it is still an option that many ophthalmologists find useful.
One newer option from Quidel, SMTube Strip Meniscometry Tube, offers an easier, faster and more comfortable way to achieve the results sought from the Schirmer test. The SMTube measures tear volume and is only minimally invasive. It can be done within 5 seconds, compared with 5 minutes for a Schirmer test. No anesthetic is needed.
As you and your staff become more comfortable with these initial dry eye diagnostic tools, consider adding others to the mix. For instance, the Sjö serum test (Bausch + Lomb) can help identify Sjögren’s syndrome based on the presence of certain biomarkers. This test can help patients avoid many years of time and effort pinpointing a diagnosis, as Sjögren’s syndrome diagnosis typically takes 3 years from the onset of symptoms, according to the Sjögren’s Syndrome Foundation.
It’s good medicine to treat DED. Now that we have better diagnostic technologies as well as better in-office and at-home treatments, we can help make patients happier and achieve better surgical outcomes. OM
REFERENCES
- Trattler WB, Majmudar PA, Donnenfeld, ED, et al. The prospective health assessment of cataract patients’ ocular surface (PHACO): The effect of dry eye. Clinical Ophthalmol. 2017;11:1423-1430.
- Chotikavanich S, de Paiva CS, Li de Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203-3209.
- Hessen M, Akpek EA. Dry eye: An inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9:240-250.
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15:539-574.
- RPS InflammaDry sensitivity and specificity was compared to clinical truth in RPS clinical study: protocol #100310.









