SightLife hosts “women advocates trip” to Nepal
Through SightLife, 117 Nepalese women were trained to treat corneal abrasions as part of its blindness prevention program.
By Robert Stoneback, associate editor
SightLife will host 11 visitors in Nepal this fall to demonstrate its efforts to eliminate corneal blindness in the country and raise funding for future programs around the world.
SightLife’s “women advocates trip” will take place in October, a little over a year removed since SightLife began its blindness prevention program in Nepal last September and in India earlier this year. SightLife inherited the Nepal program from the Francis I. Proctor Foundation for Research in Ophthalmology, based out of the University of California, San Francisco, which was completing a three-year clinical trial in the area.
“AN OUNCE OF PREVENTION …”
To date, SightLife’s Nepal program has trained 117 community health workers who have backgrounds in child and maternal health. These Nepalese women are trained to identify and treat corneal abrasions and ocular trauma. SightLife’s work is also overseen and supported by Nepal’s Bharatpur Eye Hospital and Seva Foundation.
In the year since SightLife began the program, the health workers have seen an average of 250 patients a month, according to SightLife CEO Claire Bonilla. Of those patients, 50% are treated for corneal abrasions, and 98% of those abrasions are healed within four days through antibiotic treatment.
“Prevention is one of the biggest levers we have” to eliminate corneal blindness, says Ms. Bonilla. Every person a healthcare worker saves from blindness, she adds, saves a community from the long-term health and financial impact of caring for a visually-impaired person.

EMPOWERING LOCAL WOMEN
Josie Noah, SightLife vice president of global strategy and programs, visits Nepal every three months to oversee progress on the program. One of the remarkable things about the program, she says, is how it has “elevated” the women in these Nepalese communities. With their previous training, they were primarily treating women and children, but now “feel very empowered … because it allows them to provide service to their entire community,” says Ms. Noah.
She recalls one woman in particular who was able to treat her daughter who suffered a corneal abrasion while harvesting rice. The mother provided topical antibiotics, and her daughter was healed and had perfect vision again within three days.
FUNDING PLANS
The visitors on the advocates trip are women ophthalmic leaders, says Ms. Noah. Their backgrounds include surgery, finance and human resources. OM’s Business Development Manager Abigail Markward will attend as well. Each attendee has pledged to raise $10,000 to support expansion of SightLife’s corneal blindness prevention program; SightLife is currently investigating partnerships for expansion into additional parts of Nepal and India, as well as China, says Ms. Noah.
To donate to SightLife’s blindness prevention work, visit http://bit.ly/2KDjeXq . OM

Studies: Kamra inlay safe, effective for presbyopia treatment
Two retrospective studies examined 2,969 Kamra patients who simultaneously underwent another refractive procedure.
By Robert Stoneback, associate editor
Two recent retrospective studies demonstrated the Kamra corneal inlay’s safety and effectiveness in treating presbyopic patients.
The Kamra (CorneaGen), approved by the FDA in 2015, had previously only been studied for use in restoring reading vision, according to Phillip Hoopes, Jr., MD, one of the study authors. The two new studies show that presbyopic patients undergoing a refractive procedure such as LASIK or PRK can simultaneously have a Kamra implanted to improve their sight without concerns of safety or vision quality.
Dr. Hoopes, of Hoopes Vision in Draper, Utah, helped author a retrospective U.S. study in 2017, along with Majid Moshirfar, MD, of Hoopes Vision and Luke Rebenitsch, MD, of ClearSight Center in Oklahoma City, Okla. The retrospective study followed 126 Kamra patients who had the inlay implanted simultaneously with LASIK or PRK to treat presbyopia. The results found that “it is just as effective to place a Kamra with simultaneous LASIK or PRK as it is to perform Kamra as a stand-alone procedure as the FDA originally approved,” says Dr. Hoopes.
The other new study was conducted by Japanese physician Mitsutoshi Ito, MD, PhD, of Shinagawa LASIK Center in Tokyo. Between May 2011 and January 2014, Dr. Ito treated 2,843 patients with LASIK followed by implantation of the Kamra inlay after a week or more. The patients were followed for more than four years afterwards; in that time, fewer than 7% of the study patients had the inlay removed, which includes those necessitated by development of diseases such as early cataracts, epiretinal membrane and glaucoma.
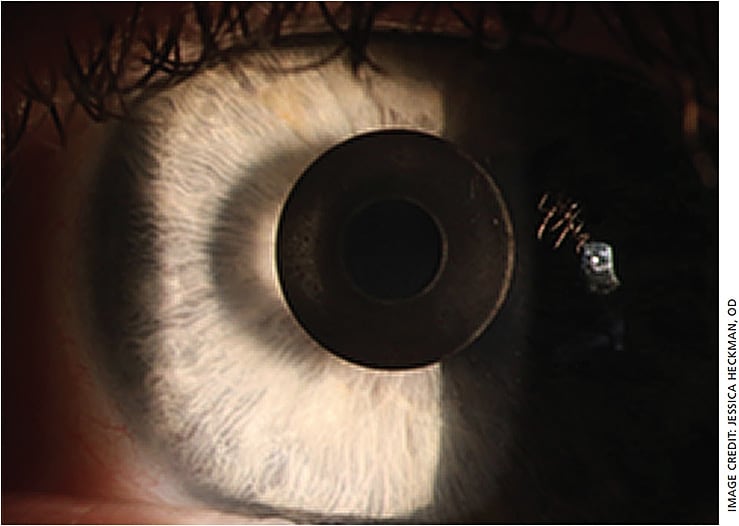
The Kamra procedure is “a much improved surgery, even in the first three years” of commercial use in the United States, says Dr. Hoopes. He compares it to the increases in quality seen by LASIK as doctors became more accustomed to it in the 1990s. Dr. Hoopes says he is even considering getting a Kamra himself when he becomes presbyopic.
The improvements with Kamra have come in part from surgeons sharing their results and developing new techniques. These include the Kamra granting improved results if it is inserted deeper within the cornea or that slightly myopic patients have better long-term results from Kamra, he says. “In time, I hope that we will see improved healing response as doctors optimize the postoperative steroid regimen and use of other anti-inflammatory medications.” OM
CORRECTION:
Our July 2018 Spotlight on Technology and Technique column incorrectly stated that the ianTECH miLOOP was available overseas. The miLOOP is not currently available outside the United States. Ophthalmology Management apologizes for the error.
QUICK BITS
The Hydrus microstent, from Ivantis, has been approved by the FDA for the treatment of mild-to-moderate primary open-angle glaucoma during cataract surgery. The FDA approved the Hydrus after the global, 556-patient HORIZON trial. Of those in the Hydrus group, 77.2% saw a greater than 20% unmedicated IOP reduction at 24 months.
Ophthalmic instrument distributor Lombart Instrument acquired Enhanced Medical Services, a U.S. distributor of pre-owned ophthalmic instruments. The acquisition “gives our organization the opportunity to work more aggressively within a doctor’s budget,” says Scott Lombart, chief commercial officer of Lombart.
Allergan announced two positive Phase 3 clinical trials for the anti-VEGF abicipar. The trials, SEQUOIA and CEDAR, examined both the eight-week and 12-week treatment regimens of abicipar. Both trials were identical and found abicipar met its safety and efficacy endpoints, compared against other treatments, in neovascular AMD patients.
Topcon received FDA clearance for its Pattern Scanning Laser Trabeculoplasty (PSLT) proprietary software. PSLT is used in a tissue-sparing laser treatment to reduce IOP in open-angle glaucoma patients. The program is cleared for use with the Synthesis and TwinStar models of Topcon’s pattern scanning lasers.
Alcon launched two new products. The Ngenuity 3D Visualization System with Datafusion software offers 3D visualization of the back of the eye and integrates with Alcon’s Constellation Vision System platform for vitreoretinal surgery. The Finesse Sharkskin ILM Forceps have a large grasping platform and texturized tip surface to assist in grasping and peeling the internal limited membrane.
Topcon launched its Topcon Augmented Reality App on the Apple Store and Google Play. By using the app, customers can hold their smart phones over a Topcon Image of the Month ad and be able to view a recording of the featured physician speaking about his or her particular case. Users can view the Topcon augmented reality experience at bit.ly/2r5P8Ey .








