For several decades, blepharitis, meibomian gland dysfunction (MGD) and dry eye disease (DED) have been thought to be three distinct entities. Blepharitis is thought to be two entities, namely anterior and posterior. Similarly, MGD is thought to be obvious and non-obvious. Not to be outdone, evaporative dry eye is thought to be distinct from aqueous insufficiency.1
To further muddy the discussion, subtle distinctions separate posterior blepharitis and MGD while many feel they represent the same entitity.2 What about chalazia, ectropion, entropion and floppy eyelid disease? Is it possible that all these diseases come from blepharitis; are they linked by the same pathogenic mechanism?
A new theory suggests that these diseases are all connected by a single concept, bacterial biofilm formation,3 and that a new term, dry eye blepharitis syndrome (DEBS), would be a more accurate and appropriate terminology. The term explains the relationship between bacterial biofilm formation on the eyelid margin and connects blepharitis, MGD and dry eye as linear changes related to biofilm maturation and migration.4
BACTERIAL BIOFILM OVERVIEW
How can a bacterial biofilm be related to blepharitis, MGD, dry eye, chalazia, ectropion, entropion and floppy eyelids? First, we must understand that bacteria come in two forms: planktonic, which are individual, free-floating bacteria, and biofilm-dwellers, which are bacteria that produce and live in a polysaccharide biofilm.5 The prevailing habitat for bacteria is within a biofilm that they create for themselves. Biofilm is a well-hydrated matrix of bacterial glycocalyx and is produced by the vast majority of bacterial species, including Staphylococci.6 It can be thought of as a layer of protective armor. Antibiotics cannot penetrate it, iodine prep can’t penetrate it and our white cells cannot penetrate it.7
Biofilms occur anywhere moisture and nutrients exist on a surface.8 The lid margin’s moisture, nutrients and warmth make it the perfect environment for a thriving bacterial biofilm. In fact, it would be unrealistic to suggest that a biofilm does not exist on the lid margin. Our biofilm probably begins forming when the lids first become colonized with lid flora. The biofilm may be well developed as early as 4 years of age as evidenced by changes in MGD evident in that age group.9
Bacteria communicate with one another within a biofilm using a chemical called homoserine lactone (HSL).10 While the HSL concentrations in the biofilm of a 4-year-old child are low, and hence the biofilm is usually non-pathogenic, the biofilm of a 50- or 60-year-old has had decades to thicken and increase its bacteria load and HSL concentrations.
Once the bacterial colony senses that its numbers have reached critical mass though an increased concentration of HSLs, quorum-sensing gene activation (QSGA) occurs.11 This happens when the bacterial population reaches a certain quorum, triggering dormant genes to activate. The newly activated genes now begin expressing inflammatory virulence factors, such as lipases, proteases and cytolytic toxins, among a host of others (Figure 1).12
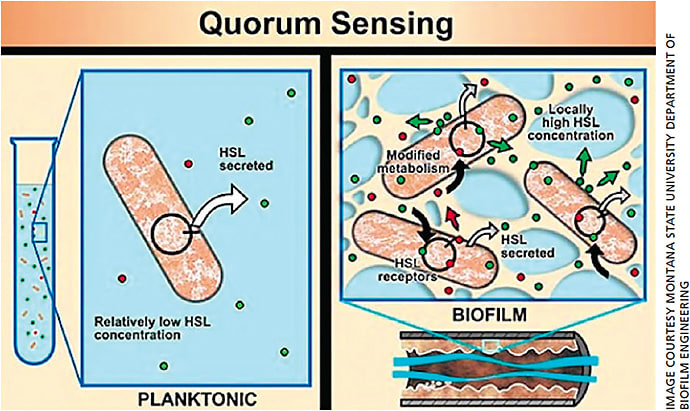
This often occurs in our 60s and 70s and mirrors the rise in incidence of blepharitis and ocular surface disease.
It is important to realize that not all strains of Staphylococcus are identical. Some are much more pathogenic than others. Some may create an early mature biofilm together with highly inflammatory toxins, producing severe blepharitis and chalazion in an 8-year-old. Others may produce minimal biofilms with relatively mild virulence factors over a person’s lifetime, therefore sparing an 80-year-old of any significant lid margin disease. Much of the variation in temporality most likely depends on the particular strain of Staphylococcus and the individual’s innate immune response.13
STAGES OF DEBS
How does the biofilm affect the ocular surface? Stage 1 of DEBS starts with a folliculitis, or inflammation and edema of the lash follicles. It always occurs first due to the easy access of the encroaching eyelid margin biofilm along the lash. As this is the beginning of DEBS, it coincides with the increased frequency of anterior blepharitis occurring in younger patients. This stage of DEBS occurs rapidly in contact lens wearers.14
In Stage 2, the biofilm continues to grow and DEBS impacts the MGs, leading to inflammation. Due to the size of the MG relative to the lash follicle and the small ductule with constant efflux of lipids, it takes longer for biofilm to accumulate and thicken within the MGs. First, a simple plugging of the MG with altered meibum (raises melting point) reduces the quantity and quality of the meibum, sometimes referred to as non-obvious MGD with minimal inflammation. As the biofilm thickens within the MG, it eventually undergoes quorum sensing and virulence factor production, which causes the inflammation that is referred to as “posterior blepharitis.”
At this point, domes of meibofilm (altered meibum) often begin to cover each meibomian ductule. It has long been thought that these little cream-colored domes over the MG were “caps” of keratin. However, because the posterior lid margin consists of non-keratinized stratified squamous epithelium, this is more likely meibofilm (altered meibum). (Figure 2)
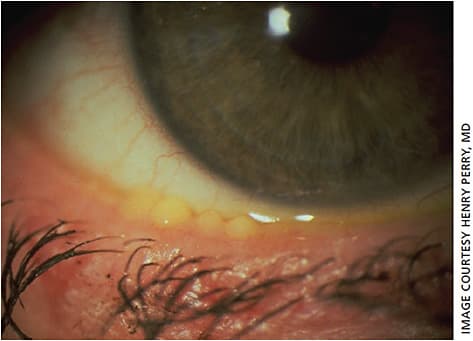
As the thickened meibofilm accumulates within the MG, the gland eventually reaches capacity. It may leak through the wall of the gland, causing a chalazion. This is a critical point in the pathogenesis of chalazia and explains why the most effective therapy is not incision and drainage or intralesional steroids but is conservative therapy.15,16 The column of meibofilm gets forced up the ductule in an effort to escape the gland. Unfortunately, it encounters a 20- to 50-year-old biofilm already present that has sealed off the orifice. The meibofilm bulges up against this original biofilm but cannot escape. Interestingly, the only therapy for this pathogenic change that is agreed upon universally is lid hygiene (more below).16
During MG probing, the practitioner may feel the penetration of this original biofilm as the probe “pops” through, which may restore some MG function.17
In Stage 3 of DEBS, the continued march of the biofilm leads to inflammation of the accessory lacrimal glands of Krause and Wolfring. This always occurs after MGD, which is easy to understand if one reviews the lid anatomy and location of these tear glands. The glands of Wolfring are located along the top of the tarsal plate and the glands of Krause deep within the fornices. These two areas are quite distant from the margin, delaying access to a growing biofilm. However, biofilms can “seed” new areas by constantly dispersing tiny bits of biofilm into their environment — the tear film in this case. If this happens hundreds of times a day for 30 to 50 years, it is not inconceivable that a microscopic bit of biofilm eventually reaches these glands.
We regularly see patients present with watery eyes, difficultly reading for long periods, burning, etc, and they are typically difficult to refract because the vision changes with every blink. A low TBUT, few if any meibomian puddles and occluded ducts confirm the diagnosis of evaporative DED. But, explaining that to the patient can be difficult, as they can’t comprehend how they can have dry eye with tears running down their cheeks. These patients with diseased MG and healthy lacrimal glands are the most common form of DED.
However, how many times do we see a patient present with the exact opposite scenario: lots of lipids, perhaps a drop of oil running down the cheek with no aqueous. How many times do we see healthy MGs together with aqueous insufficiency? Except for Sjogren’s syndrome, which is an entirely separate disease process from lid margin disease, this is a presentation we never really encounter.
If aqueous insufficiency were truly a separate overlapping disease, wouldn’t we would expect to see it present occasionally prior to MGD. We never do, because it is a later stage of the same disease — it does not happen because the morphology and anatomical location of the tear glands preclude it from happening. Lastly, inflammation keeps happening and eventually weakens the structure of the tarsus, leading to ectropion, entropion and floppy eyelid syndrome. All of these changes are a result of biofilm progression.
THERAPEUTIC OPTIONS
We have always felt lid hygiene should be the cornerstone of any therapy of chronic blepharitis. While advocating salt-water soaks may seem superfluous in this day and age, they still work.16 At the physician-patient conference, the physician explains the pathophysiology to the patient and clarifies the importance of lid hygiene. From there, the burden of treatment falls to the patient, which is important in a chronic, incurable disease.15 Lid hygiene should begin and be taught in early childhood.
Because we know this is a biofilm problem, we now have reason to attack the biofilm, which we can accomplish through several available therapeutic options. Avenova (NovaBay) is a topical spray that acts as an antiseptic, like betadine, as it kills 99% of bacteria.19 Overall, use of Avenova (hypochlorous acid) has been successful in many of our patients with DEBS. We prescribe it twice daily for one month and longer if we see continued improvement. Also, early use of this product may significantly decrease late problems.
Microblepharoexfoliation (MBE) (BlephEx, Rysurg) directly impacts the lid margin biofilm. We stress to our patients that it is another form of lid hygiene and is analogous to the dentist cleaning plaque off teeth. We use MBE in most of our patients with DEBS and especially those with Demodex and cylindrical dandruff (Figure 3).20,21
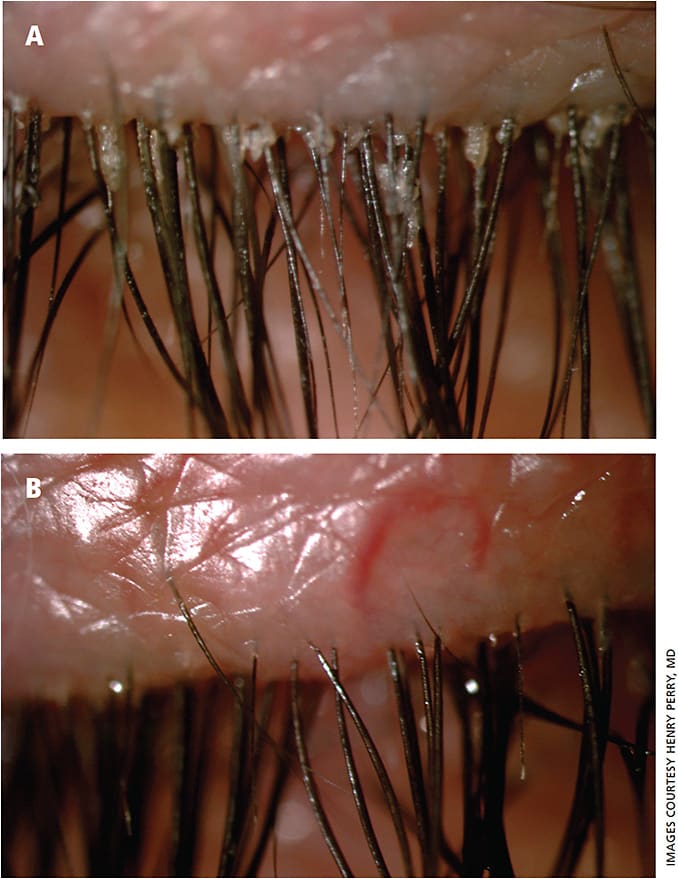
Lid scrubs (saline) may be difficult for the patient to access. I always use these first, but patients who are looking for a more convenient option can benefit from newer over-the-counter commercial agents, such as Cliradex (Bio-Tissue) and Ocusoft Lid Scrub. Unfortunately, sometimes these may lead to toxicity.22
Other treatment modalities directed at the impacted meibum, such as LipiFlow (J&J Vision), may be effective in certain patients depending on the degree of MG changes shown on meibography.23 Drugs and nutritional supplements have a place in our armamentarium as well.24,25 Drugs such as Azasite (azithromycin ophthalmic solution, Akorn) and doxycycline hyclate may be helpful adjuncts in recalcitrant cases. Although somewhat controversial, there are clear data that fish oil supplements help treat meibomian gland disease. Lid massage is effective and various adjuncts that may increase efficiency are available but may be painful.26 For cases that fail these remedies, Maskin probing may be helpful.15
CONCLUSION
Try rethinking dry eye as a biofilm problem. This will lead to earlier and more effective treatment paradigms with improved outcomes. OM
REFERENCES
- Lemp MA. Pathophysiology and diagnosis of tear film abnormalities. Int Ophthalmol Clin. 1973:13;191-197.
- Dougherty J, McCulley J. Comparative bacteriology of chronic blepharitis. British Journal of Ophthalmology. 1984;68:524-528.
- Lappin-Scott H, Burton S, Stoodley P. Revealing a world of biofilms—the pioneering research of Bill Costerton. Nat Rev Microbiol. 2014;12:781-787.
- Rynerson J, Perry HD. DEBS a Unification theory for Blepharitis, Meibomian Gland Disease and Dry Eye Disease. Clin Ophthal. 2016;10:2455-2467.
- Christersson LA, Zambon JJ, Genco RJ. Dental bacterial plaques. Nature and role in periodontal disease. J Clin Periodontol. 1991;18:441-446.
- Brothers KM, Nau AC, Romanowski EG, Shanks RM. Dexamethasone diffusion across contact lenses is inhibited by Staphylococcus epidermidis biofilms in vitro. Cornea. 2014;33:1083-1087.
- Monroe D. Looking for chinks in the armor of bacterial biofilms. PLoS Biol. 2007;5:e307.
- Absalon C, Van Dellen K, Watnick P. A communal bacterial adhesin anchors biofilm and bystander cells to surfaces. PLoS Pathog. 2011;7: e1002210.
- Viswalingam M, Rauz S, Morlet N, Dart JK. Blepharokeratoconjunctivitis in children: diagnosis and treatment. Br J Ophthalmol. 2005;89:400-403.
- Zhang C, Zhu S, Jatt AN, Zeng M. Characterization of N-acyl homoserine lactones (AHLs) producing bacteria isolated from vacuum-packaged refrigerated turbot (Scopthalm maximus) and possible influence of exogenous AHLs on bacterial phenotype. J Gen Appl Microbiol. 2016;62;60-67.
- Hastings JW, Greenberg EP. Quorum sensing: the explanation of a curious phenomenon reveals a common characteristic of bacteria. J Bacteriol. 1999;181:2667-2668.
- Bzdrenga J, Daudé D, Rémy B, et al. Botechnological applications of quorum quenching enzymes. Chem Biol Interact. 2017;267:104-115.
- Edwards AE, Bowden MA, Brown EL, Laabei M, Masey RC. Staphylococcus aureus extracellular adherence protein triggers TNFα release, promoting attachment to endothelial cells via protein A. PLoS One. 2012; 7: e43046.
- Killpartrick MR. Disposable lens risk factors and posterior lens surface contamination Cont Lens Anterior Eye. 2016;39:400.
- Perry HD, Serniuk R. Conservative therapy for chalazia. Ophthalmology. 1980;87:218-221.
- Lindsley K, Matsumura S, Hatef E, Akpek EK. Interventions for chronic blepharitis. Cochrane Database Syst Rev. 2012 16;:CD005556.
- Maskin SL. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea. 2010;29:1145-1152.
- Romero JM, Biser SA, Perry HD, et al. Conservative treatment of meibomian gland dysfunction. Eye Contact Lens. 2004;30:14-19.
- Romanowski EG, Stella NA, Yates KA, Brothers KM, Kowalski RP, Shanks RMQ. In vitro evaluation of a hypochlorous acid hygiene solution on established bbiofilms. Eye Contact Lens. 2018 Jan 15. [Epub ahead of print]
- Murphy O, O’Dwyer V, Lloyd-McKernan A. The efficacy of tea tree face wash, 1, 2-Octanediol and microblepharoexfoliation in treating Demodex blepharitis. Cont Lens Anterior Eye. 2018;41:77-82.
- Epstein I, Donnenfeld ED, Stuber R, Garf K, Perry HD. Evaluation of Claridex-Microblepharoexfoliation (Blephex) for Treatment of Demodex Blepharitis. Ophthmol (in press).
- Qui Ty, Yeo S, Tong L. Satisfaction and convenience of using terpenoil-impregnated eyelid wipes and teaching method in people with blepharitis. Clin Ophthalmol. 2018;12:91-98.
- Greiner JV. A single Lipiflow Thermal Pulsation System treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res. 2012;37:272-278.
- Perry HD, Doshi-Carnevale S, Donnefeld ED, Solomon R, Biser SA, Bloom AH. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea. 2006;25:171-175.
- Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. The effect of oral re-esterified Omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35:1185-1191.
- Griener JV. Efficacy of a 12-month manual thermomechanical regimen vs. a single automated treatment for meibomian gland dysfunction. Poster PO324 Monday 13 2017 Amer Acad of Ophthalmol.









