The FDA approved the Xen gel stent (Allergan) for the treatment of refractory glaucoma in patients who failed previous surgical treatment or in patients with primary open angle glaucoma who are refractory to maximum tolerated medical treatment. The FDA approval was granted on November 28, 2016, and the first implants in the United States, post FDA approval, occurred as soon as February 2017.
Since that time, hundreds, if not thousands, of implants have been used to treat patients with glaucoma in the United States. This microinvasive glaucoma surgery (MIGS) is the first FDA approved ab-interno method for creating a new subconjunctival drain. The Xen Glaucoma Treatment System consists of the Xen 45 Gel Stent preloaded into a Xen injector. While the Xen 45 gel stent has been a great addition to our surgical “bag of tricks,” it is not without its challenges and complications. Since its approval, we have learned a tremendous deal about methods to optimize success, safety, and surgical outcomes.
This article will provide a brief overview of the implant and surgical technique, the evolution in the use of mitomycin C (MMC), and the role of needling in the postoperative phase, as well as review the major clinical studies evaluating Xen in the management of open-angle glaucoma.
Surgical Technique
The Xen 45 is a 6-mm-long gel stent, with a 45-micron inner-diameter lumen and a 210-micron outer diameter. It is made of a porcine gel material that is cross-linked with glutaraldehyde. The implant is delivered by a novel injector (with a 27-gauge double bevel needle) via an ab-interno method through a 1.8-mm clear corneal incision (Figure 1).1 In my hands, Xen 45 performs best when 1 mm of the implant is in the anterior chamber, 2 mm in the scleral wall, and 3 mm in the subconjunctival space (Figure 2). Having the implant a bit longer in the subconjunctival space allow the implant to lay flat against the scleral wall. It also allows for the implant to be needled a bit more easily, if that becomes necessary, as you have more of the implant to work with. Of note, the company recommends the following proportions: 1 mm in the anterior chamber, 3 mm in the sclera, and 2 mm in the subconjunctival space.
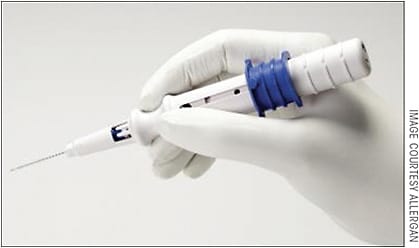
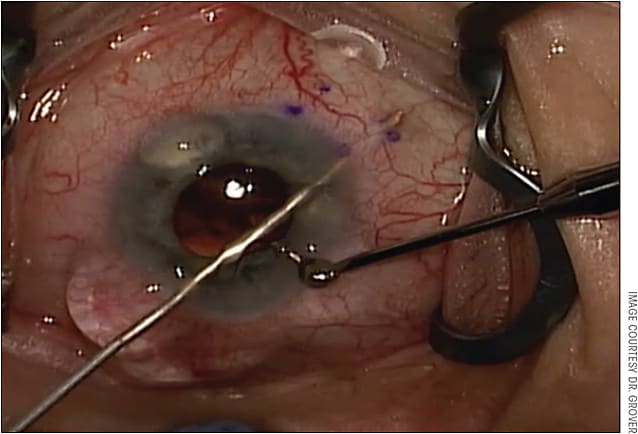
When implanting the Xen 45 through the 1.8-mm corneal incision, there should be a 1-mm paracentesis roughly 90 degrees away, through which the second instrument can be injected to provide counter traction, as it does require a reasonable amount of force to pass the 27-gauge needle tip of the injector through the scleral wall (Figure 3). One of the biggest initial challenges is getting used to the injector and the sliding mechanism. Fortunately, surgeons can gain familiarity with the mechanics of the injector in the practice lab, the mandatory training session the company provides to surgeons.
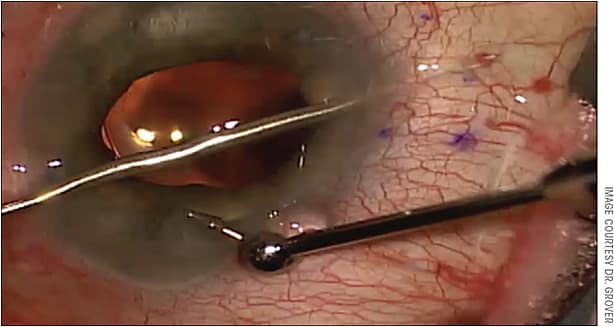
Another big challenge for me was avoiding a “flick” during implantation of the stent. As you slide the blue handle forward on the injector, 2 things occur: 1) the first 50% of the handle slide allows the Xen 45 to be pushed into the proper location, and 2) the second 50% of the handle slide causes the needle to retract into the injector and leaves the gel stent in place. If a surgeon is placing any kind of torque on the globe with the second instrument, he or she will immediately experience a “flick” as the needle from the injector disengages from the scleral wall and slides fully into the injector.
To avoid this issue, surgeons must be aware of the second instrument and allow the globe to return to its orthotropic position before sliding the handle forward and disengaging the eye. The moment I notice the needle beginning to retract after the stent has been fully deployed, I allow the eye to return to its resting position and remove my second instrument from the eye. I then use my nondominant hand to stabilize the injector while fully advancing the slider forward. The video accompanying this article, available online, shows my nondominant hand stabilizing the tip of the injector after I remove my second instrument from the paracentesis. This technique allows one to avoid the flick and damage the implant and/or iris.
Xen implantation - MMC post injection from Pentavision on Vimeo.
One must be advised, however, that although placement of the implant may appear to be simple, it is relatively challenging and can quickly become complicated if not done appropriately. Taking extra time in the practice lab and ensuring one is comfortable with the injector with both hands is essential (as is going through a proper training program with the company).
Evolution of the Use of Mitomycin C
One of the attractive and unique aspects of the Xen 45 is the ab interno delivery mechanism, which eliminates the requirement for a conjunctival incision. Because of the minimally invasive approach, the Xen 45 implant is now possibly the safest and least invasive method for creating a new drainage system into the subconjunctival space. Because the device still shunts aqueous into the subconjunctival space, subconjunctival fibrosis and scarring is a concern. To date, the best way to modulate wound healing following this filtration surgery is with MMC.
During the FDA trial, the investigators were mandated to take down the conjunctiva to apply MMC with sponges. However, since FDA approval, surgeons have been able to use discretion when it comes to method and amount of MMC use. During my first 6 months of experience with Xen after FDA approval, I would inject 20-80 mcg of MMC into the superior quadrant in the subconjunctival space as far posteriorly as possible. I would then roll the MMC into the superior nasal quadrant, exactly where I was planning on placing the Xen implant (Figure 4). While my colleagues and I felt this technique was effective, it exposed the superior quadrant to MMC possibly without need.
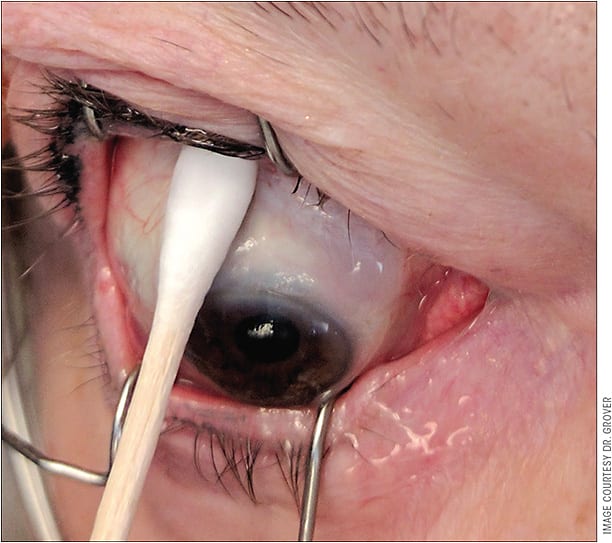
Over the past 6 months, my method of MMC application has changed slightly. While I still have the eye pressurized with a viscoelastic, I rotate the globe inferiorly and inject MMC (20 mcg to 40 mcg) as far posteriorly as possible, in an area just posterior to where the implant will be shunting aqueous fluid (Figure 5). I now implant the Xen 45 stent first. I believe this is more effective and direct and does not expose the patient to additional risk.
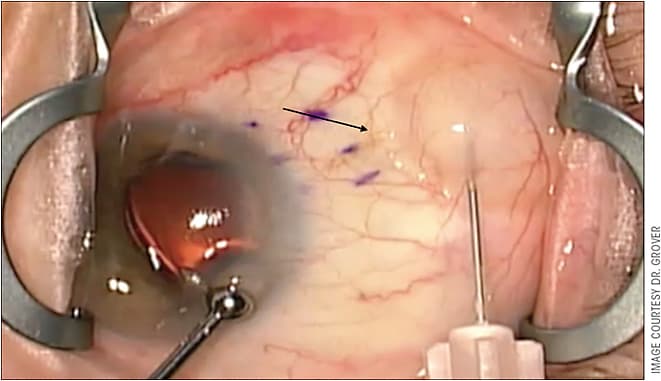
The concern with this method is that MMC will reflux into the stent and into the anterior chamber. I avoid this by taking several precautions. First, I ensure the anterior chamber is filled with viscoelastic and the IOP is supraphysiologic prior to giving the MMC injection. Second, I aggressively irrigate out the viscoelastic, which also allows a bleb to form, subsequently diluting the MMC in the subconjunctival space. Lastly, the patient leaves the operating room with a supraphysiologic pressure thus ensuring fluid is still leaving the Xen 45 implant for at least another 10 minutes to 15 minutes. After using this technique for the past 6 months in my patients, I have not seen any sign of MMC toxicity. Additionally, I have experienced improved bleb morphology and improved IOP control. I am in the process of evaluating my outcomes before and after this switch.
Needling
Needling is an essential component to managing the Xen 45 implant in the postoperative phase. In my hands, as well as in other data sets, roughly 30% to 40% of patients require needling with MMC.2 My basic approach is to create a solution of 0.2 mL of 0.2 mg/mL of MMC mixed with 0.8 ml of 2% preservative-free lidocaine, in a 1 mL syringe. Depending on the case and my perception of the patient’s scarring potential, I will inject anywhere from 0.2 mL to 1 mL of this mixture into the far posterior subconjunctival space, directly posterior to the implant. After 10 minutes to 15 minutes, I then use a 30-gauge needle and enter the subconjunctival space 2 mm to 3 mm from the tip of the implant in a tunneled fashion. I then use the tip of the needle to break the scar tissue away from the posterior and anterior aspect of the implant. I also try to bring the tip of the implant anteriorly so that it sits on top of the pocket of scar tissue where it initially was located.
One of the greatest stimuli for subconjunctival fibrosis in the postoperative period is aqueous in the subconjunctival space that contains inflammatory mediators. As such, I avoid needling within the first month and wait until the anterior chamber and the conjunctiva have completely quieted down before attempting needling. My experience is that needling within the first few weeks causes more scar tissue to form and subsequent failure.
Data
The results of the FDA trial involving the Xen 45 microstent demonstrated that the implant was able to reduce IOP and medication use without causing any unexpected safety concerns when compared to traditional glaucoma surgery.1 Specifically, the study demonstrated that at 12 months, 75.4% of patients had a ≥20% reduction in IOP from baseline on the same or fewer medications. The mean IOP decrease from baseline was 9.1 mmHg (observed data) and the mean decrease in medications was 1.6. These data must be taken with a grain of salt because as previously mentioned, patients in this study had the Xen 45 implant placed after taking down the conjunctiva to apply MMC. In practice, most surgeons are not performing a conjunctival dissection and thus, the surgery is less traumatic. Theoretically, this less invasive approach should correlate with a better surgical outcome. I have seen this in my practice and am in the process of evaluating our group’s surgical outcomes with Xen.
Schlenker et al performed a retrospective, multicenter, international, interventional cohort study evaluating standalone Xen 45 vs trabeculectomy. This study was not able to detect a significant difference in safety or efficacy outcomes between these 2 surgeries and reported success rates roughly similar to previously reported prospective data on trabeculectomy outcomes. Additionally, 43% of eyes that underwent a Xen 45 implant required needling.2 This study did not evaluate issues such as cost effectiveness of each surgery, number of patient visits, or impact of dose of MMC on surgical outcomes.
More real world data, that are not company sponsored, are required and will be coming in the next few months to years. In my experience, outcomes from this surgery will depend heavily on surgical technique, amount and placement of MMC, needling technique, and postoperative management. These statements are true following trabeculectomy surgeries as well.
Conclusion
The Xen 45 implant is a novel MIGS for the treatment of refractory open-angle glaucoma. The available studies demonstrate that this surgery is as safe and effective as traditional glaucoma surgeries.
One must realize that Xen 45 surgery is a real glaucoma surgery that has the potential for real complications (as do all incisional glaucoma surgeries). Wound healing and subconjunctival fibrosis are still a challenge, and they impact the success of this surgery. Most importantly, this surgery still creates a bleb, but the morphology is characteristically low, posterior, and diffuse, which is more ideal. When performing this surgery, one still needs to be comfortable managing a bleb as well as performing a needling procedure.
The big questions about the Xen 45 that remain unanswered relate to long-term safety outcomes, the results of noncompany sponsored data, the success in various glaucoma subtypes and ethnic backgrounds, the cost, and the long-term integrity of the device in the real world. Despite these (and other) concerns, I am optimistic about the Xen 45 implant and have generally had very good success using it to surgically manage my patients with refractory open angle glaucoma. I think it is a great addition to the glaucoma surgical armamentarium and a very efficient, safe, and predictable method for creating subconjunctival filtration. GP
References
- Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months.” Am J Ophthalmol. 2017;183:25-36.
- Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124(11):1579-1588.









