LaserACE
By Mitchell A. Jackson, MD, and AnnMarie Hipsley, DPT, PhD
Presbyopia is derived from the Greek words présbys, meaning old, and ōps, meaning eye. Since this word describes the progressive loss of accommodative ability that comes with age, classifying presbyopia into stages allows for a targeted and evidence-based treatment.
The three stages of presbyopia, based on lens opacities and higher order aberrations (HOA), together comprise dysfunctional lens syndrome.1
- DLS stage 1. The lens is rigid, losing flexibility. Presbyopia and HOA are apparent.
- DLS stage 2. Advanced presbyopia and HOA. There is loss in contrast sensitivity, increased light scatter and the lens shows early opacity.
- DLS stage 3. Opacity in the lens is significant and affects activities of daily living. This stage corresponds with cataracts.
Loss of accommodation with age is normally attributed to an increasingly rigid lens. Thus presbyopia has typically been treated along the visual axis with spectacles, contact lenses, IOLs or corneal surgery. Their collective goal: to increase the range for which vision is sufficient by increasing the depth of focus (DOF) or “pseudo-accommodation.” This can be done by inducing small pupils with inlays, inducing monovision, changing the zonal optics with bifocals, or changing the spherical aberration with multifocals.
However, expanding pseudo-accommodation by treating along the visual axis comes with trade-offs. Small pupil treatments can reduce visual function, monovision could decrease intermediate visual acuity, and bifocal and multifocal treatments can result in reduced image quality. Also, these treatments do not attempt to restore true dynamic accommodation.
Thus, the need remains for a treatment that can both expand pseudo-accommodation in our patients without trade-offs and restore true dynamic accommodation.
THE OCULAR RIGIDITY FACTOR
In addition to the lenticular influence on accommodation, new research has identified the influence of HOA and extralenticular structures, such as the zonules, choroid and sclera, on accommodative ability.2,3 Ocular rigidity, as well as a decline in accommodation, increases with presbyopia progression and also correlates with increasing age.4 This indicates a possible likewise correlation between presbyopia and ocular rigidity.
Focused, multifactorial treatment applications that mediate the effects of HOA, extralenticular structures and ocular rigidity on accommodation function have advantages over lenticular-based treatments. The primary advantage is that ocular optics are not manipulated, and the cornea, native lens and the visual axis remain unaffected. Patients in DLS stage 1 are excellent candidates for treatments that do not affect the visual axis.
In this stage, patients have: reduced accommodative ability; very little opacity; or other age-related damage affecting their near vision.
OBJECTIVE MEASUREMENT
We can objectively measure visual performance using wavefront aberrometry. With this technology, we can measure patients’ HOA, pseudo-accommodation, true accommodation and effective range of focus (EROF). The EROF is the range of vision with acceptable blur and is equal to the sum of the true accommodation plus the pseudo-accommodation. We can predict patient visual acuity from wavefront aberrometry using a metric known as the Visual Strehl of the Optical Transfer Function (VSOTF). The VSOTF is complex and includes information about visual performance such as refraction, and quality of vision, such as astigmatism, coma and HOA. A VSOTF of 0.3 correlates to a visual acuity of approximately 20/20, while a VSOTF 0.12 correlates to a visual acuity of approximately 20/32. VSOTF is defined as:5
VSOTF = the area under the contrast sensitivity-weighted optical transfer function
the area under the contrast sensitivity-weighted optical transfer function for a diffraction-limited eye
Quality of vision, including retinal image quality and visual perception, is largely influenced by spherical aberrations. Positive spherical aberration occurs when incoming peripheral light rays bend too much, while negative spherical aberration occurs when incoming peripheral light rays do not bend enough. Spherical aberration in the lens is negative when the lens is in an accommodated position or in a more convex shape.6 Spherical aberration in the lens is positive when the lens is in a dis-accommodated position, assuming a less convex shape. In a young eye, positive spherical aberration of the cornea is mostly balanced by the negative spherical aberration in the crystalline lens, which results in optimal vision. However, in the aging eye, negative lens spherical aberration shifts toward positive, which reduces contrast sensitivity and visual function to below what it could be.7
DOF DETERMINATIONS
To determine objective DOF, the VSOTF is computed as a function of defocus to create a through-focus curve (Figure 1). The diopter range between the two points on the curve where the VSOTF is at a threshold value is the objective DOF . A threshold value of 50% of the maximum VSOTF is typical and is used for all the presented data. Surgeons can determine the DOF for both near and distance and, by plotting the near and distance through-focus curves together, surgeons can determine the EROF, true accommodation and pseudo-accommodation.

The diopter range of the near and distance DOFs is the EROF. Figure 2 is an example of two different wavefront scans (iTrace ray-tracing aberrometer, Tracey Technologies) and through-focus curves at near (blue) and distance (pink) for a young patient who can still accommodate. This patient had an EROF of 3.17 D, true accommodation of 2.19 D and pseudo-accommodation (EROF minus true accommodation) of 0.98 D. This is expected for a typical young eye.
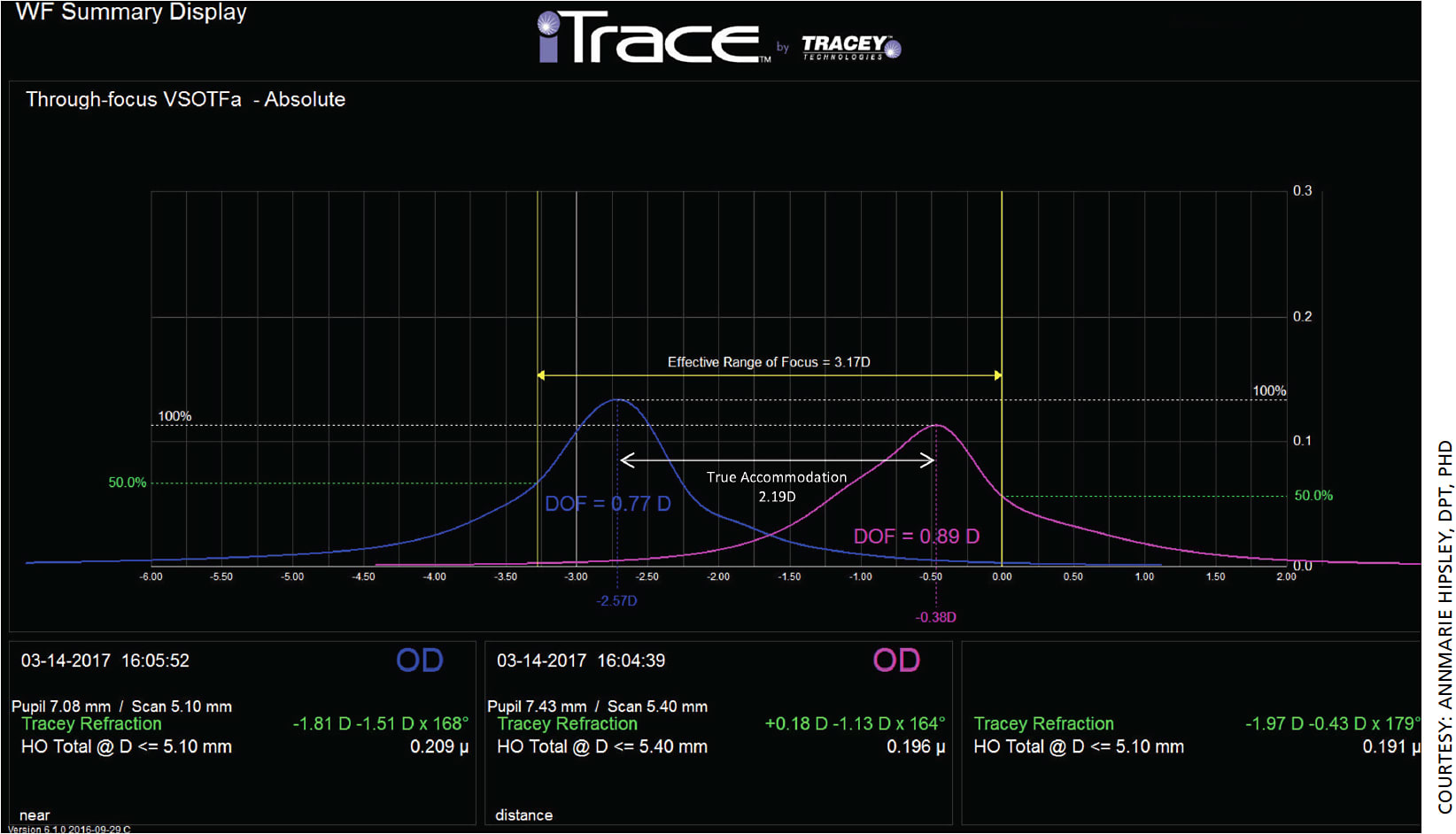
For a typical presbyopic eye, on the other hand, we would expect reduced EROF and no true accommodation. Figure 3 depicts two different wavefront scans and through-focus curves at near (blue) and distance (pink) for a typical presbyopic 59-year-old patient who has not undergone any presbyopic treatment.
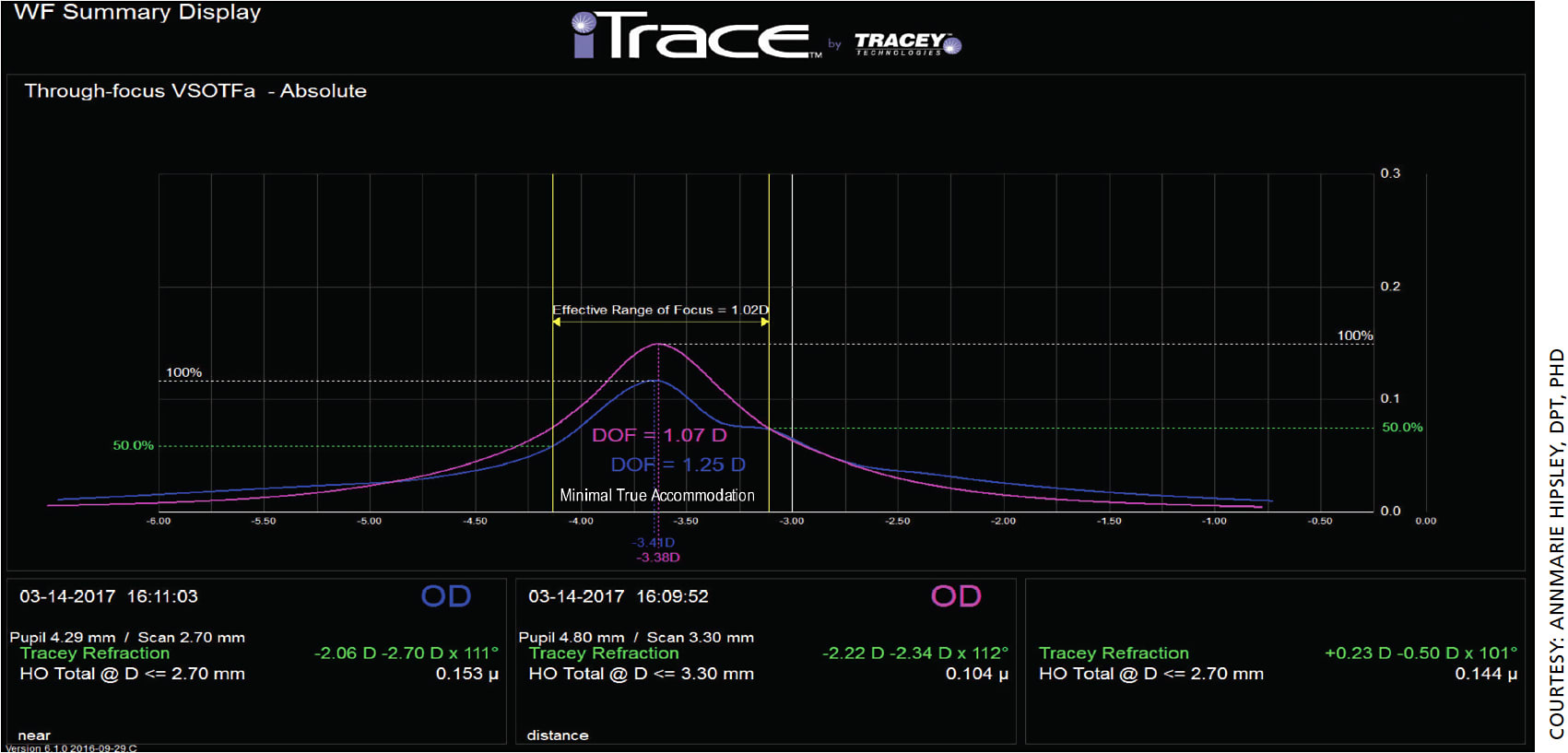
In this example, the EROF is reduced compared to the young patient (at 1.02 D), and there is no true accommodation as expected. A treatment that could demonstrate an improved EROF without directly manipulating optics would enhance DOF and improve visual acuity in DLS stage 1 patients who still have a clear, movable lens.
TREATING DLS
Laser anterior ciliary excision (LaserACE, Ace Vision) is an eye laser therapy designed to reduce ocular rigidity and restore both dynamic accommodation and enhance pseudo-accommodation. It is an investigative device, not approved in the United States.
As our eyes age, the connective tissues continuously crosslink. For example, this crosslinking results in progressive biomechanical stiffness of the fibrils and microfibrils in the sclera. These fibrils and microfibrils increase overall ocular rigidity, which creates stress on the underlying accommodative structures and leads to dysfunction.8,9 LaserACE creates micro-ablations (micropores) via an Er:YAG laser, used on the sclera directly, to produce an “uncrosslinking” effect. This restores compliance to the ocular globe and specifically over critical zones of physiological function where the extralenticular structures are attached. The LaserACE’s micro-ablations do not touch the visual axis, cornea or lens. The laser targets the sclera in three critical zones of physiological significance (Figure 4):
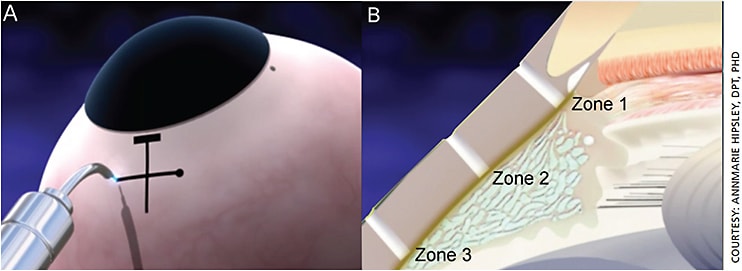
- Zone 1. The scleral spur at the origin of the ciliary muscle (0.5 mm to 1.1 mm from the anatomical limbus);
- Zone 2. The mid-ciliary muscle body (1.1 mm to 4.9 mm from the anatomical limbus);
- Zone 3. Insertion of the longitudinal muscle fibers of the ciliary, just anterior to the ora serrata at the insertion zone of the posterior vitreous zonules (4.9 mm to 5.5 mm from the anatomical limbus).
LaserACE is designed to restore biomechanical movements that are critical to the accommodative mechanism and its overall function by mitigating the restrictions’ ability to produce forces that will affect the movable lens. This mitigation results in an improvement in EROF by restoring accommodation and producing improved DOF through biomechanical enhancement of pseudo-accommodation.
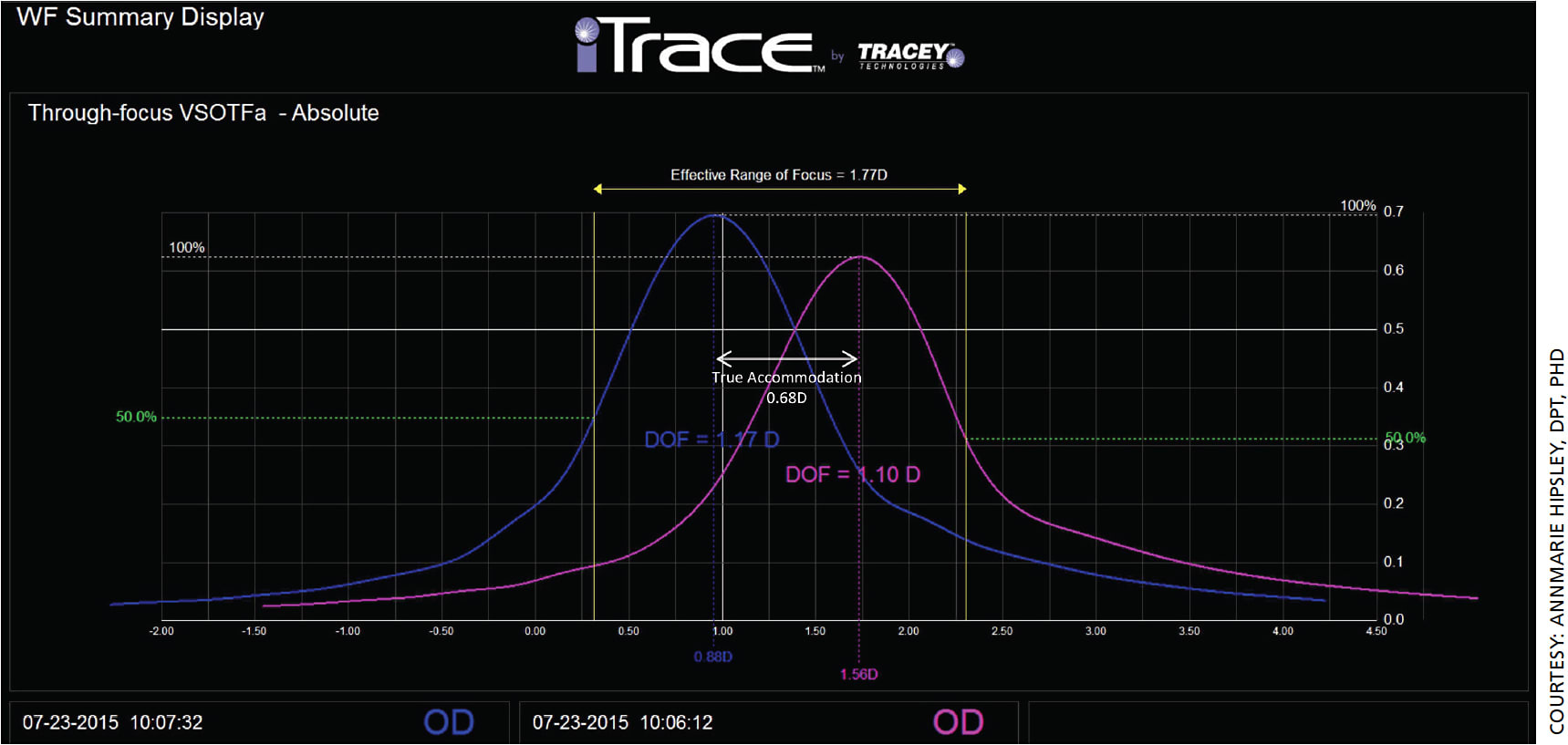
Figure 5 shows two representative through-focus curves for a LaserACE patient at near (blue) and distance (pink). The effective range of focus in this example is 1.45 D, the true accommodation is 0.31 D and the pseudo-accommodation (EROF minus true accommodation) is 1.14 D. Mean patient postoperative EROF was 0.64 D higher than mean preoperative clinical accommodation. True accommodation was evident and averaged 0.25 D, corresponding to a one-line increase in near visual acuity. Mean patient postoperative DoF also increased by 0.84 D compared to mean preoperative DoF.
The iTrace aberrometer (Tracey Technologies) was used to evaluate DLS changes in spherical aberration, coma, trefoil and defocus during dynamic accommodation in six eyes following the LaserACE procedure. The spherical aberration in LaserACE patients shifts to negative (-0.011) during dynamic accommodation. Additionally, coma (mean difference .06) appears to have the largest impact on HOA (mean difference .11) during accommodation, and trefoil the smallest impact (mean difference .0005) on HOA (mean difference .11) during accommodation. These results show that LaserACE patients have good overall quality of vision and visual function.
CONCLUSION
Wavefront aberrometry can objectively measure dynamic accommodation, differentiating true accommodation from pseudo-accommodation, and objectively measure quality of vision in DLS patients. Those in DLS stage 1 are excellent candidates for LaserACE treatment as it does not touch the visual axis. It is designed to restore true physiological dynamic accommodation and enhanced pseudo-accommodation. This represents a notable improvement in the EROF for better visual acuity, DOF and overall quality of vision for presbyopes — all without a loss in distance vision, any vision compromise or optical surprises that may be impacted with use of vision correction procedures. OM
REFERENCES
- Waring GO IV, Stahl JE. Premium cataract surgery: a step by step guide. Hovanesian J, ed. Slack Inc. 2012. p. 15.
- Croft MA, McDonald JP, Katz A, et al. Extralenticular and lenticular aspects of accommodation and presbyopia in human versus monkey eyes. Invest Ophthalmol Vis Sci. 2013;54:5035-5048.
- Croft MA, Nork TM, McDonald JP, et al. Accommodative movements of the vitreous membrane, choroid, and sclera in young and presbyopic human and nonhuman primate eyes. Invest Ophthalmol Vis Sci. 2013;54:5049-5058.
- Detorakis ET, Pallikaris IG. Ocular rigidity: biomechanical role, in vivo measurements and clinical significance. Clin Exp Ophthalmol. 2013;41:73-81.
- Thibos LN, Hong X, Bradley A, Applegate RA. Accuracy and precision of objective refraction from wavefront aberrations. J Vis. 2004;4:329-351.
- Koomen M, Tousey R, Scolnik R. The spherical aberration of the eye. J Opt Soc Am. 1949;39:370-376.
- Berrio E, Tabernero J, Artal P. Optical aberrations and alignment of the eye with age. J Vis. 2010;10:34.
- Ethier CR, Johnson M, Ruberti J. Ocular biomechanics and biotransport. Annu Rev Biomed Eng. 2004;6:249-273.
- Fazio MA, Grytz R, Morris JS, et al. Human scleral structural stiffness increases more rapidly with age in donors of African descent compared to donors of European descent. Invest Ophthalmol Vis Sci. 2014;55:7189-7198.
About the Authors
The VisAbility system is designed to correct lens dysfunction.
By Kenneth Beckman, MD, FACS
Dysfunctional lens syndrome can present in many forms. One of the earliest signs of loss of lens function is the loss of accommodation, or presbyopia. While the crystalline lens may maintain clarity and excellent correctable vision, the lens gradually loses accommodation as patients enter their forties — a source of much frustration to patients.
At the moment, no one approved procedure addresses the accommodation problem. One under investigation, however, just might.
First, a rundown of what is on the market to address presbyopia.
DESIRE AND EVOLUTION
Although multiple treatments exist that allow patients with presbyopia to read, no current treatment completely solves the problem. Among the current treatments for presbyopia are spectacle and contact lens correction. While bifocal spectacles, multifocal contact lenses and monovision may give excellent results, many patients want more: independence from glasses and contacts. This desire has led to the evolution of many surgical treatments for presbyopia, the most common of which is corneal laser refractive surgery. However, because these procedures do not correct for accommodation, the only way to allow spectacle independence is to intentionally leave one or both eyes myopic.
Other corneal procedures include various corneal inlays. Again, these procedures do not restore accommodation. Corneal inlays are placed within the central cornea in one eye to allow for near vision in that eye only, while the other eye remains focused at distance. This is not true monovision since the treated eye maintains distance vision but the near vision is still monocular.
Various lens-based procedures have also become popular, installed either at the time of cataract surgery or with a clear lensectomy. These lenses include multifocal lenses, extended-depth-of-focus lenses and pseudo-accommodative lenses.
AN IMPLANT WITH PROMISE
Currently being studied in the United States is the VisAbility Micro-Insert system (Refocus Group, Inc.). The system holds a CE mark and is available for sale and use in Europe. The procedure involves placement of four PMMA implants within scleral tunnels to expand the sclera and restore the anatomic state for accommodation.
The implants are placed in the four quadrants, between the rectus muscles. Prior versions required hand dissection of the tunnels, and therefore were often irregular in length, depth and location. Now, placement is automated, which makes it significantly easier than earlier versions. A docking station placed at the limbus stabilizes the eye and provides guidance for the scleral tunnel creation. The scleral tunnels are created using a sclerotome, which adds precision and repeatability. The tunnels are created 4 mm posterior to the limbus, with a length of 4 mm and a depth of 400 μm.
Once the tunnels are created, a two-piece implant is placed in each tunnel. The first piece is passed with a shuttle through the tunnel and a second piece is then clasped to the first from the other end of the tunnel to secure it in position. Once all four implants are placed, the conjunctiva is closed.
The unique benefits of this procedure: It is extraocular, bilateral rather than unilateral, and does not involve the visual axis so it therefore does not degrade visual quality. (Near vision typically continues to improve over time, according to data presented by company president Mike Judy at OIS in May.) The treatment is meant to be performed bilaterally at one sitting, but for the two-year, phase 3 clinical trial, both eyes were treated two to four weeks apart. Typically, the procedure takes 15 to 20 minutes per eye. The implants are removable.
THE EARLY RESULTS
The United States trial had 360 patients (14 of whom are my patients). While the complete one-year data are not available to the trial investigators, some demographics from individual sites look promising.
In a dual-site subsample, 20 patients were asked about their vision. This sample included 10 consecutive patients from both sites (40 eyes). According to company data, in distance-corrected near visual acuity, 100% of patients in this group reached J3, (20/40); 95% reached J2 (20/30); and 63% reached J1 (20/20); at 12 months. In uncorrected near visual acuity, 100% reached J3; 95% reached J2; and 90% reached J1; at 12 months.
In another single-site patient satisfaction survey involving 26 patients, when describing the change in near visual performance after surgery without glasses as compared to before surgery without glasses, 100% responded as significantly better or better at three months. At 30 months, 100% still responded as significantly better or better. As late as 36 months, 95% responded as significantly better or better and 5% responded as no change. (As for serious side effects, the company reported no major side effects, and according to Mr. Judy’s presentation, there were no long-term complications.)
CONCLUSION
With our aging population, the search for a treatment for presbyopia continues to grow. While several options are already available, the VisAbility Micro-Insert system, if ultimately approved in the United States, would provide a bilateral, extraocular, reversible treatment that spares the visual axis to preserve visual quality at all distances. The effect seems to improve over time and has shown a high level of patient satisfaction to date. OM











