Quick Hits
CMS issues sterilization clarification
Ophthalmic groups win a break for ASCs.
By René Luthe, Senior Editor
In an update to an edict issued in 2014, CMS now says that the practices of most ophthalmic ASCs would be in compliance with its rules for instrument sterilization. The statement clarifies CMS’ mandate from last fall that ASCs could no longer use immediate-use steam sterilization (IUSS) on a routine basis. That policy appeared to require that ASCs acquire and use terminal sterilization units and possibly acquire many more sets of instruments, necessitating significant capital outlay.
In response to the 2014 policy, the American Academy of Ophthalmology (AAO), the Outpatient Ophthalmic Surgery Society and the American Society of Cataract and Refractive Surgery met with CMS on two occasions to express their concerns and to educate the agency on the etiology of toxic anterior segment syndrome and endophthalmitis. The groups also showed the agency the results of a recent survey of ASCs’ current sterilization and instrument-cleaning practices.
The problem, according to Michael X. Repka, MD, the medical director of governmental affairs for the AAO, was one of semantics. “CMS had understood ‘immediate-use steam sterilization’ to refer to the antiquated ‘flash’ sterilization’ rather than the commonly practiced short-cycle steam sterilization,” he explains. Medical organizations previously used the term “flash” to describe sterilization cycles for instruments and devices that were not packaged in preparation for sterilization, to be subject to minimal drying time and immediate use. CMS recorded unsafe practices and adverse events — including surgical-site infections — in health-care facilities where the term “flash” sterilization was used.

Michael X. Repka, MD, is the medical director of governmental affairs for the American Academy of Ophthalmology.
“Nothing has changed beyond the terminology, as we continue to recommend that ophthalmic ASCs follow directions from both instrument and sterilization manufacturers,” Dr. Repka says.
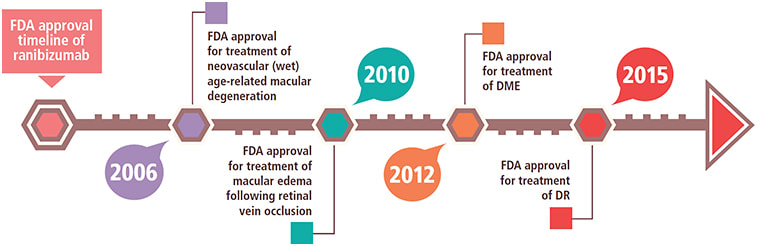
Lucentis approved for diabetic retinopathy treatment
The FDA approved ranibizumab (Lucentis, Genentech) for the treatment of diabetic retinopathy (DR) in diabetic macular edema (DME) patients. FDA approval followed results from the RISE and RIDE Phase III clinical trials, two identically designed, parallel, double-masked, sham treatment-controlled trials. Three groups totaling 759 DR and DME patients were randomized to receive monthly treatment with 0.3 mg ranibizumab, 0.5 mg ranibizumab or sham injection. After 24 months, color fundus photography showed a three-step or better improvement in ranibizumab groups compared to the sham group, and the trials demonstrated safety comparable to previous studies.
Brave new “Tweeter”
Wills Eye embraces Twitter to connect with patients
By René Luthe, Senior Editor
Last month, Wills Eye Hospital took another cyberstep into the ever-expanding world of social media to reach patients with a Twitter chat on “Health and Beauty Tips for Your Eyes.” The hour-long chat hosted by oculoplastics surgeon Jacqueline Carrasco, MD, addressed “the safest cosmetic surgical and non-surgical options available for good eye health and facial rejuvenation,” according to Wills Eye, including injectables, blepharoplasty, laser resurfacing and skin cancers.

Dr. Carrasco says it was her first experience with the online social networking service. “I’m not even registered on Twitter, so it’s an absolutely new modality for me! I think it’s quite an interesting way for patients to connect to their doctors.”
The chats were the idea of the Wills Eye media department, she reports, who set up the hashtag (#WillsEyeCares) and advertised it to those who follow the Wills Eye Twitter sites.
As much as Dr. Carrasco enjoyed the interaction with prospective patients, she says it had its challenges. Two media department staff members manned computers, typing her answers to questions. These were projected onto a screen for her to read. “The hardest part was trying to formulate my response in 140 characters,” Dr. Carrasco says of the maximum permitted in a Twitter response. “To answer medical questions or explain a procedure, I had to be very succinct. Occasionally we did … two Tweets to explain one answer.”
Though her answers were by necessity brief, she believes they were adequate for the medium. “I don’t think people on Twitter want very long responses. It was a way to get a quick question answered, things like, ‘Is Botox good for everyone?’” And Dr. Carrasco did manage to get around Twitter’s brevity requirements by “Tweeting” links to videos on Wills Eye’s website, including one on blepharoplasty and some before-and-after photos of oculoplastics patients. “To prepare, I had put together some things on our website that would explain procedures better than a 140-character response,” she says.
Despite the help she needed as a newbie, Dr. Carrasco believes smaller practices could hold their own Twitter chats with a little preparation and social-media savvy. “If you have Twitter followers, you could certainly tweet out that you are going to discuss a certain topic. It’s something the doctor could probably do from home,” she says.
It was the Philadelphia-based hospital’s second foray into Twitter, following an earlier chat on macular degeneration.
QUICK BITS
Nikon Corp. has agreed to acquire Optos, a retinal imaging firm, for $400 million (259.3 million pounds) in cash. Under terms of the agreement, Nikon will pay 340 pence a share in cash for Optos, a 30.5% premium to the closing price at time of the proposed acquisition. Optos specializes in ultra-widefield retinal imaging technology.
Carl Zeiss Meditec launched the Humphrey Field Analyzer (HFA) 3. The perimeter, which uses the company’s Liquid Lens technology, has a Liquid Trial Lens, and is designed to offer the appropriate refractive correction from measurement information entered by the user. For more information, visit www.zeiss.com/hfa3.
Iridex received FDA approval for its Cyclo G6 Laser, which is used to treat glaucoma and its symptoms. The laser system will be sold with two disposable delivery probes, the MicroPulse P3 probe and the G-Probe. Additional probes could be introduced in the coming year, the company says.
Shire’s SHP607, a protein replacement therapy designed to prevent retinopathy of prematurity, received the FDA’s Fast Track designation and is currently undergoing a Phase II study, the company says.
Clompus Consulting Group, LLC has launched a digital service to help ECPs communicate with potential patients using their own voice. For more information, visit www.clompusconsulting.com. OM
CORRECTION
In the January 2015 issue on p. 10, Ophthalmology Management erred in saying that the figures referred to cataract surgeries; they referred to the number of those with cataracts. OM regrets the error.








