Quick Hits
Trying to stop ICD-10 in its tracks
The Oct. 1 deadline nears, but its critics won’t quit.
By Zack Tertel, Senior Editor
As practices prepare to officially transition to ICD-10 on Oct. 1, two recently introduced bills aim to block or alter the billing and diagnostic codes.
On April 30, Texas Congressman Ted Poe introduced the Cutting Costly Codes Act of 2015. If passed, the bill would prohibit the federal government from implementing, administering or enforcing regulations that would replace ICD-9 with ICD-10.
In addition, the cleverly named ICD-TEN Act, or Increasing Clarity for Doctors by Transitioning Effectively Now, was introduced on May 12 and sponsored by Tennessee Republican Rep. Diane Black. While details are unclear, Rep. Black’s plan is to institute an ICD-10 transition period rather than push back the deadline or eliminate ICD-10, says Paul Larson, MBA.
Mr. Larson, a senior consultant with Corcoran Consulting Group, says he is not sure that either has a chance of passing. However, the ICD-TEN Act’s transitional aspect makes it the more likely of the two to have a chance, although some of details in this bill that allow for lower specificity codes are still murky, he says.
“You have to get into the details of whether or not you can get away with less specific ICD-10 coding, and that’s not entirely clear in this bill,” Mr. Larson says.
These bills cite the disruption to health-care providers that would result in a transition to ICD-10. Also, practices face additional implementation costs. For example, the total cost for a typical medium-sized practice is estimated to range between $213,364 to $824,735, according to a Nachimson Advisors 2014 study. A medium practice is defined as 10 physicians, one full-time coder and six administrative staff. The costs include pre-implementation, including training, vendor/software upgrades and testing, as well as post-implementation, including productivity loss and payment disruption costs.
While these bills suggest a need for ICD-10 reform, not everyone is convinced that changing the planned ICD-10 implementation date is necessary. Count the American Hospital Association (AHA) on the pro-ICD-10 side. The AHA released a February 2015 statement in which it “strongly supports the Oct. 1, 2015 ICD-10 compliance date and opposes any steps to delay this implementation date.” The AHA’s ICD-10 Factsheet also maintains that a potential dual coding system running ICD-9 and ICD-10 codes simultaneously is “unworkable.”
Also, further delay may be unnecessary as readiness improves, Mr. Larson says. “There are much harder things out there than ICD-10,” he says. “When people actually get trained, they seem to say, ‘We can do this.’”
Even if the Cutting Costly Codes Act of 2015 is successful, the process to implement ICD-11 is already underway and a first draft is expected in 2017, according to the World Health Organization (WHO). However, the transition from ICD-10 to ICD-11 is expected to be a smaller scale change than ICD-9 to ICD-10, Mr. Larson says, which would make for an illogical and more complex transition from ICD-9 to ICD-11.
With the Oct. 1 deadline quickly approaching, each passing day decreases the chance of ICD-10 being delayed.
“We’re less than four months from implementation,” Mr. Larson says. “It gets more and more unlikely that there is going to be any delay.”
PQRS reporting made easier for EHR-less practices
IRIS Registry wins 18 new measures, CMS reaffirmation.
By René Luthe, Senior Associate Editor
According to the American Academy of Ophthalmology, CMS is penalizing an estimated 30% of physicians with a 1.5% cut this year for failing to report Physician Quality Reporting System (PQRS) data in 2013. And the penalties are getting worse: Penalties rise to 2% in 2017 for physicians who don’t satisfactorily report their quality data for 2015, so help with correct reporting is becoming an emergent issue for practices.
Thus CMS’ recent approval of 18 new clinical quality-reporting measures for the Academy’s IRIS Registry is welcome news indeed, as is CMS’ reaffirmation of the registry’s designation as both qualified for submitting PQRS data and as a Qualified Clinical Data Registry (QCDR). The 18 new clinical quality measures are aimed at practices that have not yet implemented electronic health records (EHR). Also, the measures correct the previous problem of few PQRS reporting options for ophthalmic subspecialties by addressing clinical outcomes for retina, cornea, oculoplastics, glaucoma, uveitis and pediatric ophthalmology. The Academy worked with subspecialty societies to develop the measures, which it says should help subspecialty members meet requirements for PQRS and avoid program penalties.
The qualified registry designation means practices may use the IRIS registry to collect and report clinical data for PQRS and cataracts measures, whereas the QCDR designation means that users can report on quality measures across multiple payers and are not limited to Medicare beneficiaries or measures within PQRS.
As for practices that don’t yet have EHR, the new clinical quality measures provide help when they use an alternative QCDR reporting method, in which they submit data themselves using the IRIS Registry. Now, those practices can choose from the new IRIS Registry those clinical quality measures as part of the nine required for PQRS. The Academy offers additional information on PQRS — 2015 deadlines. Reporting options and measures, as well as instructions for using the IRIS Registry for PQRS reporting — at www.aao.org/aaoe/practice-management/pqrs/index.cfm.
Do you measure up?
A new index aims to estimate the efficiency of eye-care practices.
By René Luthe, Senior Editor
Researchers, including ophthalmologists, at various University of California schools of medicine, business and School of Public Health have developed an efficiency index that estimates the performance of an ophthalmologist’s practice as a function of cost, number of patients receiving care and quality of care.
They undertook the project, they wrote in “Index to estimate the efficiency of a ophthalmic practice” in JAMA’s May 28 online journal, as part of a broader effort to address the disparity between this country’s high health-care spending and relatively poor outcomes.
U.S. health-care spending comprised 17.3% of GDP in 2011 and was projected to grow by 8.4% last year as the Affordable Care Act required increased coverage, they noted. To accommodate expanded coverage while reducing costs, the researchers designed an efficiency metric that reflects the ratio of quality to cost per patient, thereby enabling measurement of the impact of specific reforms on the health-care system, as well as comparing the efficacy of each.
Investigators examined 36 ophthalmic subspecialty practices from October 2011 to September 2012 at a university-based eye-care institute.
They expressed the efficiency index (E) as E = b(Na/Ca)Qy, a function that included adjusted costs (Ca), adjusted number of patients (Na) and quality (Q).
Constant b limits E between 0 and 1, and constant y modifies the influence of Q on E. The median adjusted number of patients was 5516, the median adjusted cost was 1.34, the median quality was 0.89, and the median value of the efficiency index was 0.26.
“The results of the efficiency index could be used in future investigations to determine [their] sensitivity to detect the impact of interventions on a practice,” the researchers concluded. For more on the study, go to http://tinyurl.com/pdkh4xf
Agency, AAO, are quantifying extended depth of focus
FDA and the Academy form task force to put specs on EDOF.
By Karen Blum and Martin Fox, MD
U.S. ophthalmic regulators are gearing up for a new generation of extended depth of focus (EDOF) intraocular lenses. Last spring, the American Academy of Ophthalmology established a task force, with FDA participation, whose aim is to ensure that these lenses pass through newly created systems designed for rapid review. The devices are designed to enhance intermediate and possibly near vision without the halos and light scatter associated with diffractive bifocal lenses.
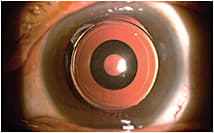
Figure 1: The Acufocus IC-8 small aperture IOL is designed to provide excellent visual quality across a broad range of vision for
presbyopic patients with cataracts.
COURTESY: ROBERT ANG, MD
EDOF lenses take in all light rays before spreading them out over an extended range. “The point of focus is not as crisp as a monofocal lens but at the same time it gives you a little more depth of focus so intermediate vision is improved,” says Jack T. Holladay, MD, MSEE, chair of the task force and a clinical professor of ophthalmology at Baylor College of Medicine in Houston. AMO’s Tecnis Symfony and AcuFocus’ IC-8 received approval in 2014 for use in Europe. Symfony, in a phase 3 FDA clinical trial here, may be approved by 2017, says Richard Lindstrom, MD, founder and attending surgeon of Minnesota Eye Consultants.
There will be more. Dr. Holladay expects all multifocal lens makers to offer an EDOF lens within two years.
Redefining the meaning of premium
By Martin Fox, MD
Until recently, cataract/implant patients have been offered three less-than-perfect intraocular lens options to address reading issues after surgery: monofocal aspherics either targeted for plano outcomes or monovision; multifocal diffractive; and accommodating varieties. Each implant variety has inherent drawbacks. They require the surgeon to carefully select an implant based on an intimate understanding of the patient and his visual goals. The issues involved and the vagaries of potential outcomes missing treatment targets make these choices difficult — and are why many surgeons avoid choosing premium lenses for their cataract patients, preferring to stay within the comfortable confines of monofocal aspheric lenses.

Figure 2. The Tecnis Symfony IOL’s diffractive echelette design elongates the eye’s focus and results in a continuous, extended range of vision.
Yet, these lenses do not address reading correction; they avoid quality of vision issues such as halo and glare associated with light-splitting diffractive multifocals, along with the uncertainty of outcomes associated with existing forms of accommodating lenses.
Wouldn’t it be nice to have an intraocular lens implant that offers reliable near-vision function without compromising distance vision?
The future approaches
As the quality and precision of cataract surgery improves with the use of femtosecond laser technology and we move into an era of refractive cataract surgery, there is demand for better lens implant technology addressing patient demands for improved distance and near-vision function. This demand has stimulated the rise of a host of new lens entries for FDA scrutiny. Newly engineered designs attempting to emulate true accommodation include PowerVision’s FluidVision* and the Akkolens Lumina; however, reliability and effectiveness still await evaluation.
To address this near-vision issue, ophthalmic specialists are designing lenses with an enhanced depth of focus (EDOF) over a wider range of vision; these lenses have improved near function without unduly compromising the quality of distance acuity. The interest in this new approach has led to the formation of the combined FDA and the AAO effort to create a task force to facilitate faster approval of this new lens modality as their characteristics fall outside of conventional modes of evaluation. A new standardized set of tools and guidelines that define the characteristics of an EDOF lens has been established. EDOF entries must create at least one-half diopter more depth of focus than a monofocal lens at working distance of 66 cm and, further, lenses must test appropriately to a defined defocus curve to demonstrate effectiveness.1
What’s within reach
Abbott Medical Optics’ Tecnis Symfony lens has recently received CE mark approval and is being studied in U.S. trials as well. This IOL maintains a full range of constant, high-quality vision after surgery, with a rate of halo and glare comparable to a standard monofocal lens.2 The surface diffractive design results in 50% less light loss than traditional diffractive technologies by neutralizing chromatic aberration.3,4
Following the lead of the photographic community in creating better quality lenses, the Symfony lens corrects for the differential of color focus to extend the range of vision with enhanced contrast. The result is sustained mean visual acuity of 20/20 through 1.5D of defocus and 20/40 through 2.5 diopters with halo and glare levels comparable to conventional monofocal lenses. The design also appears to increase tolerance of residual spherical and astigmatic refractive errors +/- 0.50 and up to 1.00D of astigmatism.
Another EDOF entry is the AcuFocus IC-8. This hydrophobic acrylic lens makes use of the same small aperture optics at work with the KAMRA corneal inlay lens incorporating a 3.23-mm, 5-micron thick opaque mask with 1.36-mm aperture. The lens has demonstrated remarkable function over a wide defocus curve similar to the KAMRA corneal inlay (AcuFocus, Irvine, CA). IC-8 IOL patients show continuous functional vision of 20/40 or better over a 4.0D range.5 When paired with a small amount of myopia of -0.75D to -1.00D, the IC-8 IOL improves visual acuity (as compared to conventional implants) over a larger range of intermediate and near distances, with minimal effect to distance vision.6 Cataract surgeons may elect to enhance prior monofocal cataract post-op patients with a pocket placed KAMRA inlay in the nondominant eye to enhance depth of focus and reading.
REFERENCES:
1. Personal Communication, Jack Holladay, MD.
2. DOF2014CT0002, Data on File, Symfony Harmony EMEA Trial, Abbott Medical Optics. Presented at European Society of Cataract & Refractive Surgeons (ESCRS) Annual Congress, Sept. 13, 2014, in London.
3. TECNIS Symfony DFU, Z310939 Rev. 03 Revision Date: Oct. 3, 2014.
4. Shrader, CG. Innovative IOL Breaks New Ground in Presbyopia Correcting Implant Technology. Ophthalmology Times. October 2014.
5. Salamonis L. New IOLs in the US and Beyond. Ophthalmology Management. December 2014.
6. Vukich et al. Achieving Reliable and Predictable Results with a Small Aperture Inlay. 2014 AcuFocus white paper.
WHY THE TASK FORCE
The FDA was concerned about a backlog in approvals of new medical devices including IOLs, and wanted to put guidelines and tools in place to expedite approvals, says Anne Coleman, MD, PhD, a professor of ophthalmology at the University of California-Los Angeles’ Jules Stein Eye Institute. Ophthalmology was one of the first areas of scrutiny, she says. Despite an increase in the number of premium IOL submissions to the FDA, information on FDA guidance documents and recognized standards were limited regarding acceptable adverse event benchmarks and appropriate test methods that were applicable to particular premium IOL devices.1 As a result, the agency had been evaluating premium IOL submissions on a case-by-case basis, with significant resources spent on repeat submissions.
A joint AAO/FDA workshop held in March 2014 brought together ophthalmologists, including Dr. Coleman, researchers, industry representatives and regulatory officials to go over endpoints and assessment methods for premium IOLs. From there, the task force was established.
“We want to be able to offer patients the latest technologies,” Dr. Coleman says, but with the assurance that manufacturers followed safe, effective testing procedures, and “if they say they’re providing intermediate vision, that they do so.” Many patients are opting for cataract surgeries at younger ages, and expect improved vision, she says. “There’s a limited window for error.”
DEFINING AND DECIDING
Over the past year, the task force has developed guidelines that define what an EDOF lens is, and what testing is necessary, Dr. Holladay says. Interim documents from the group, to be sent to IOL manufacturers this summer for feedback, list three qualifications for EDOF lenses:
1. Its peak performance BSCVA must be less than one line different from the monofocal control based on a sample providing a 95% confidence interval of 100 eyes.
2. The defocus curve for the EDOF of IOL needs to be 0.5D greater than the defocus curve for the monofocal IOL control at logMar 0.2 (20/32).
3. EDOF lenses must have at least 50% of eyes BSCVA or better than or equal to logMAR 0.2 (20/32) at 67 cm.
The group also has been defining the testing standards for these premium IOLs, including defocus curve testing and acceptable tilt/decentration measurement techniques, Dr. Holladay says. A final guideline is expected to be compiled by June 2016, he says, and will be submitted to an AAO journal for publication and posted to the FDA website so manufacturers can use it as a resource.
“This was a big thing for the FDA, and volunteering ophthalmology has been seen as a big success,” Dr. Coleman says. “It really opened the door for quicker [device] approvals.” OM
Dr. Lindstrom is a consultant for Alcon, AMO, AcuFocus, and Bausch + Lomb.
REFERENCE:
1. Lum F. et al. Special Commentary: Food and Drug Administration and American Academy of Ophthalmology Sponsored Developing Novel End Points for Premium Intraocular Lenses Workshop. Ophthalmology. Published online April 18, 2015. DOI: http://dx.doi.org/10.1016/j.ophtha.2015.02.038.
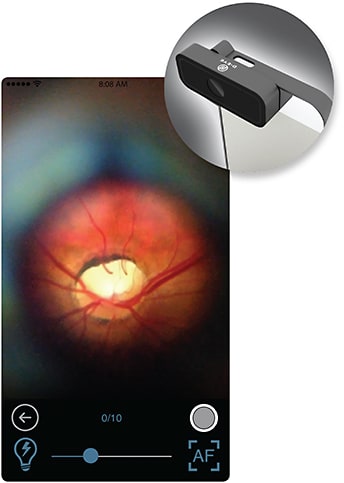
Your smart phone can get smarter
The D-Eye fundus camera can assess retinal health.
Professor Minas Coroneo, a researcher and faculty member at the University of New South Wales, was eyeing D-Eye at this year’s ARVO conference. The D-Eye is a two-inch by one-inch (approximate) retinal fundus camera that attaches to a smartphone, be it iPhone or Android. According to its inventor, ophthalmologist Andrea Russo, MD, of the Università di Brescia, the D-Eye has an agreement ratio to a slip lamp biomicroscope of 90% for detecting diabetic retinopathy. Its field of view, according to the D-Eye web site, is 20° (http://www.d-eyecare.com/). Dr. Russo sees his D-Eye meeting myriad evaluation challenges faced daily by many providers: primary care physicians; those treating children; optometrists; veterinarians; and more.
Dr. Russo, standing amid the hubbub of people asking questions at the D-Eye booth, says he decided to create the camera soon after he got out of residency. So many people were facing diabetic retinopathy and ocular hypertension. He wanted a way to couple the phone with a computer to assess the health of the retina.
To get the right illumination of the retina, the D-EYE controls the path of the light coming from the smartphone’s LED. In order to control light reflections and glares on the cornea, the device has polarizing filters. Moreover, a diaphragm is necessary to control this light scatter, Dr. Russo says.
The D-Eye received FDA registration last year; Dr. Russo says the camera has a patent pending worldwide. It affixes to the phone with a magnet.
Professor Coroneo brought up another reason that the D-Eye is a valuable ophthalmic — and societal — resource. He says it could attract more physicians into the field. He explained that Dr. Russo’s D-Eye costs less than an ophthalmoscope. The D-Eye is about $400, an ophthalmoscope, he says, can be a lot more. (The D-Eye took best in show at the Academy’s Eye MD Association conference last year.)
Another plus for the D-Eye, says Professor Coroneo, is that residents lose ophthalmoscopes, or don’t buy them because they’re heavy. “They can’t put them in their pocket. No medical student buys it.”
QUICK BITS
Eye diagnostic developer i-Optics secured EUR 13.7 million equity from existing and new investors during a recent financing round. The companys says it plans to use the captial to accelerate the commercialization of Cassini, a diagnostic platform used during premium refractive cataract surgical procedures. The company will also support the EasyScan eye diagnosis solution’s international market adoption through a partnership with Japanese multinational HOYA.
The Tear Film & Ocular Surface Society (TFOS) announced it has raised the necessary funds to produce a sequel to the original TFOS Dry Eye WorkShop (DEWS). Sponsors include: Alcon (Title Sponsor), Shire (Platinum Sponsor), Allergan (Gold Sponsor) and Bausch & Lomb (Silver Sponsor). TFOS anticipates DEWS II’s evidence-based process to require more than 18 months to complete. For more, visit www.tfosdewsreport.org.
Correction and clarifications
In the March 2015 issue, the article entitled "Keratoprosthesis can be a viable option," figures 2 and 4b were incorrectly attributed. Figure 2 was courtesy of the Boston Kpro Department, Mass. Eye and Ear Infirmary; Figure 4b was courtesy Paschalis E, Chodosh J, Spurr-Michaud S, et al. In vitro and in vivo assessment of titanium surface modification for coloring the backplate of the Boston keratoprosthesis. Invest Ophthalmol Vis Sci. 2013 Jun 4;54(6):3863-3873.
In the May 2015 issue, the article entitled “Advances in assessing the quality of vision” should have referred to the device as the AcuTarget HD, not the HD Analyzer.
Ophthalmology Management sincerely regrets the errors.








