Increasing IOL accuracy
Why intraoperative aberrometry works for more than just the outliers, and how to achieve repeatable results.
By Samuel Masket, MD
In the earliest days of intraoperative aberrometry (IA), the technology’s value and accuracy were questioned. At the time, surgeons typically employed it for the outliers — the unusual cataract surgery cases — but did not use it routinely, in part because of the cost of the equipment, including the business model with a per-case fee. However, given that it is a noncovered expense, physicians may charge patients for its use.
Now the technology has gone through several iterations, making it faster, more accurate and easier to use. As a result, I find it useful for virtually all cataract- and lens-based surgery.
The original ORange device was upgraded to the ORA, which garnered data quicker and with increased accuracy. Next came ORA with VerifEye technology, which provided streaming real-time information. The fourth generation system is now available, called ORA with VerifEye+, which adds a dynamic reticle (Figure 1) and allows the surgeon to view the streaming refractive data in the ocular of the microscope. This eliminates the need to look at an ancillary screen during aberrometry and when positioning toric lenses or evaluating the effect of astigmatic incisions.
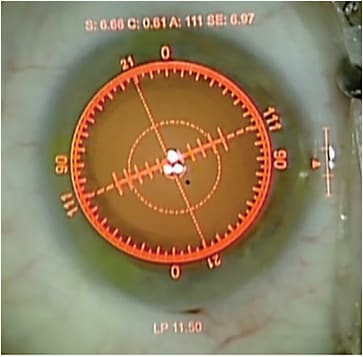
Figure 1. Intraoperative view of dynamic reticle readout. Streaming refractive error is noted above a suggested lens power seen below.
COURTESY SAMUEL MASKET, MD
Testing IA’s validity
In 2012, I conducted a study of IA. In a consecutive series of 200 patients, I nested a group who had the same IOL platform, who had no meaningful pathology, no prior corneal surgery and a best-corrected visual acuity potential of at least 20/30 — 131 of the 200 patients fit the criteria.
In the study, presented at the 2013 ASCRS annual meeting, the eye was measured preoperatively with both the IOLMaster and the LenStar and tested multiple formulae; I chose an IOL power based on the results. Intraoperatively I changed IOL power in accord with the results of the ORA.
Among the 131 cases, I changed IOL power in response to the ORA in 42.7%, whereas in the remaining 57.3% ORA and my presurgical IOL power selection were concordant. The chief outcome measure was the spherical component of the refractive error measured at three weeks after surgery. I chose the spherical component, not spherical equivalent, because toricity was not addressed uniformly.
I found that 94% of eyes in both groups were within .50D of the intended outcome, suggesting that it is necessary to change IOL power in a sizable number of patients and that ORA is useful technology. The study revealed the value of IA as it represents a significant step to getting patients closer to their expected visual outcomes. Today, I use it in nearly every case.
IA accuracy
In another recent study, we compared accuracy of intraoperative aberrometry technology and the OCT-based IOL power calculation in 39 eyes of 20 patients undergoing cataract surgery after previous laser vision correction.1 The IOL power was estimated preoperatively, and the WaveTec ORA wavefront aberrometer measured aphakic refractive measurements intraoperatively and calculated the IOL power. While IA didn’t achieve a statistically significant difference from OCT technologies, we saw a trend towards increased IOL power accuracy with more than 70% of patients within .50D in the postrefractive eye. This is an excellent outcome, particularly without having previous knowledge of their refractive correction.
Obtaining optimal results
To have success with IA accuracy, surgeons must keep several things in mind and follow a repeatable protocol. First, with a visual inspection, make sure the epithelium is not irregular, roughened or significantly dry. One must be certain the patient’s eye is not deformed by the speculum, so detect whether the drapes or the lid speculum are pulling on the eye or deforming the globe. It’s best to manipulate the patient’s head to have the cornea in the middle of the palpebral fissure, rather than positioned near either the upper or lower eyelid margin.
Another key area of successful IA involves setting IOP at physiologic levels, between 15 mm Hg and 21 mm Hg. For years I have used a surgical tonometer to set IOP prior to checking the incision for leakage at the close of surgery. But, when performing IA, I do this prior to obtaining measurements. To maintain the patient’s IOP, it’s necessary to seal the patient’s incision, but you must be careful not to overhydrate the cornea or the incisional tissue — this can induce astigmatism and distort the cornea. An alternative to stromal hydration is filling the anterior chamber with an OVD. However, there is a concern that the OVD fill could alter the ORA readings.
Achieving reproducibility
• Remove OVD with “cortical cleanup”
• Be careful with stromal hydration
• Avoid speculum deformation
• Set IOP levels between 15 mmHg and 21 mmHg with balanced saline solution
• Perform aphakic refraction two to three times
Toward that end, I along with my co-authors compared ORA readings with BSS (balanced salt solution) chamber fill and immediately sequentially filled with OVD. We evaluated six OVDs.2 In total we looked at 120 eyes. The six OVDs were: Amvisc (Bausch + Lomb), Healon (AMO) and Provisc (Alcon), Discovisc (Alcon), Amvisc-Plus (B+L) and Healon GV (AMO). There were 20 eyes in each of the six groups.
The OVDs with a higher index of refraction (Discovisc and Amvisc-Plus) had the greatest effect on lens power and gave an inappropriate reading for the IOL power. Specifically they would have approximately 0.50D error on the low side, so the patient would have a hyperopic surprise if IA was performed with an OVD chamber fill. However, the low molecular weight agents (Provisc, Healon and Amvisc), with a lower index of refraction, induced no meaningful difference, so surgeons can feel comfortable using those OVDs with IA. Results were borderline with Healon GV.
Placing toric IOLs
IA is particularly helpful in patients receiving toric IOLs, because most of us don’t go to surgery with knowledge of posterior corneal astigmatism. We go to surgery with knowledge of the eye’s anterior corneal astigmatism, but the aphakic refraction is altered by posterior corneal astigmatism; in general we don’t know for sure what the posterior cornea is contributing to the refraction, although Douglas Koch, MD, has shown that there is, on average, a half-diopter of against-the-rule astigmatism caused by the back surface of the cornea. Once we perform IA for an aphakic refraction we know the correct total astigmatism and can insert the appropriately powered toric IOL. I have found that IA is very helpful to not only determine the axis of astigmatism, but also the magnitude of astigmatism.
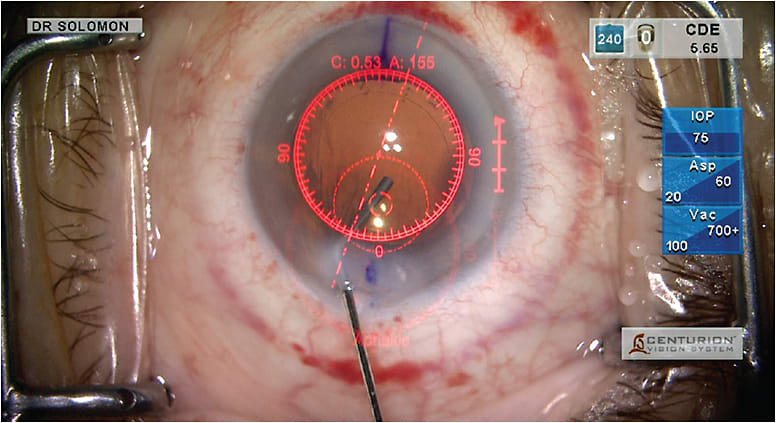
Figure 2. The VerifEye+ dynamic reticle sends live data verification directly into the surgeon’s right ocular.
COURTESY ALCON
After placing the toric lens, I remove the OVD, seal the incision and pressurize the eye. Then, with IA, we determine whether the lens needs to be rotated.
Learning IA
The learning curve for this technology is not steep or long, and I expect that surgeons should be comfortable with IA after using it on 10 patients. One issue is the reduced working space between the aberrometer and the surgical field.
Like any other surgery, IA must be performed in a logical, step-by-step fashion. Failure to control the variables, such as patient IOP or maintaining a clean corneal surface, results in unreliable readings. By following the path and staying within the parameters, one will likely obtain reproducible results.
I recommend that surgeons who want to adapt to IA track their own outcomes. Go into surgery with an IOL plan and then follow the patient’s results. After performing refraction two to three weeks later, make a comparison between the original suggested IOL power or what you planned to use and the outcome with IA. Look at the mean absolute error for several patients both with and without IA, and you should understand its value.
IA’s value
Medicare and most third-party payers do not cover refractive surgery.3 However, we try to make it an attractive option for patients. Therefore, we tend to charge a low amount so that patients will take advantage of the technology. When the patients see the results, they believe in the technology as much as I do. Keep that in mind when you decide whether to try IA and how much to charge.
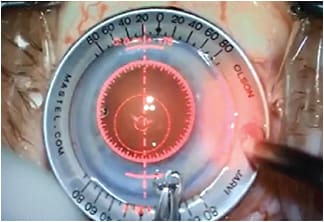
Figure 3. LRI overlay with VerifEye+. Real-time refractive information (cylinder and axis) is displayed at the top along with LRI overlays on axis, allowing surgeons to see instantaneous impact of the incisions as they are executed.
COURTESY ALCON
Surgeons have recognized the value of IA when they cannot predict lens power. However, they are unlikely to see accurate IOL power calculation results without regular use. Therefore, surgeons should be encouraged to adapt to this technology for routine cataract surgery cases as well as more difficult scenarios. OM
REFERENCES
1. Fram NR, Masket S, Wang L. Comparison of Intraoperative Aberrometry, OCT-Based IOL Formula, Haigis-L, and Masket Formulae for IOL Power Calculation after Laser Vision Correction. Ophthalmol. 2015 Mar 9. [Epub ahead of print]
2. Masket S, Fram NR, Holladay J. Influence of Ophthalmic Viscosurgical Devices on Intraoperative Aberrometry. JCRS; in Editorial Review.
3. Medicare Benefit Policy Manual, Chapter 16 §90, p 26. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/bp102c16.pdf.
About the Author | |

| Samuel Masket, MD, is in private practice at Advanced Vision Care and a clinical professor, David Geffen School of Medicine, University of California at Los Angeles.
|








