Learning to read retinal OCT
Physicians who spend time understanding OCT’s lexicon will discover its diagnostic value.
By Jessica G. Lee, MD and Richard B. Rosen, MD
Ocular coherence tomography (OCT) has become a most useful imaging test for diagnosing, treating and monitoring retinal diseases, as it provides a virtual “optical biopsy” of the macula (Figure 1). This noninvasive imaging technique produces high-resolution cross-sectional images of the retina, anterior segment, optic nerve head and retinal nerve fiber layer. OCT employs low-coherence interferometry of near-infrared light to acquire views of the eye, which resemble histological sections.
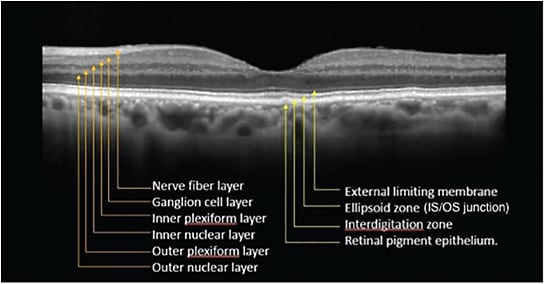
Figure 1. Spectral-domain optical coherence tomography image from the macula of a normal eye. The following retinal layers are labeled: nerve fiber layer; ganglion cell layer; inner plexiform layer; inner nuclear layer; outer plexiform layer; outer nuclear layer; external limiting membrane; ellipsoid zone (previously referred to as the IS/OS junction); interdigitation zone; and retinal pigment epithelium.
To be sure you get the most out of OCT images, first try to identify all the retinal layers as illustrated here, then identify what is abnormal and in which layer the abnormality occurs; finally, correlate that with the pathophysiology of the disease.
This article will review the indications for and interpretation of OCT when applied to retinal diseases commonly encountered by the general ophthalmologist.
CATARACT SURGERY — BEFORE AND AFTER
For the comprehensive ophthalmologist, OCT images of the macula may be particularly important in evaluating patients both prior to and following cataract surgery. With the increasing popularity of premium intraocular lenses (IOLs), patients’ expectations of excellent results are high. Preoperative OCT images of the macula can help reveal any pre-existing retinal pathology that may limit visual outcomes, allowing the surgeon to appropriately counsel a patient before a disappointing outcome occurs.
Following cataract surgery, OCT is particularly sensitive for detecting early Irvine-Gass syndrome, or pseudophakic cystoid macular edema (CME) thanks to the optical cross-section of the macula it provides, which allows for visualization of the retinal layers and its structural details. CME following cataract surgery is estimated to occur in about 4% to 40% of all cases; OCT is an easy, noninvasive way to make the diagnosis (Figure 5).

Figure 5. Spectral-domain OCT of CME after cataract surgery.
Once diagnosis of CME postcataract surgery is made, you can start treatment with topical NSAIDs and steroids, and use serial OCT images to monitor the patient’s recovery. The measurements of the central retinal thickness will help you detect worsening or resolution of macula edema. The caveat here, however, is that OCT appearance does not always correlate with visual performance.
KNOW ITS LIMITATIONS
While OCT is an excellent way to detect structural disease states, be aware that the technology does not always suggest functional abnormalities. CME is one such condition with which you will have to bear that in mind. The differential diagnosis of cystoid macular edema is extensive, including etiologies such as diabetic retinopathy, venous occlusive disease, age-related macular degeneration, medication side effects, retinitis pigmentosa and uveitic conditions.
Additional imaging with fluorescein or ICG angiography is often necessary to help identify the underlying etiology of cystoid macular edema. Furthermore, a dilated eye exam with careful evaluation of the peripheral retina is critical for detecting various occult or masquerade causes of CME such as ocular tumors or peripheral vascular lesions, which may pose a risk of significant morbidity.
Early referral to a retina specialist could offer additional treatment options, including intravitreal steroid injections or anti-VEGF drugs for cystoid macula edema that is unresponsive to topical treatment.
DIABETIC RETINOPATHY
In patients with diabetes, macular OCT can help diagnose diabetic macular edema (DME), even in patients whose clinically significant macula edema (CSME) is not readily visible on slit lamp biomicroscopy (Figure 6).

Figure 6. OCT of diabetic macular edema.
Once you diagnose DME, early referral to a retina specialist is important so treatment can be initiated promptly. The armamentarium for treating DME is growing and includes intravitreal injections of anti-vascular endothelial growth factor (VEGF) (ranibizumab, Genentech; bevacizumab, Genentech; and aflibercept, Eylea), and intravitreal injections of triamcinolone acetonide, dexamethasone and fluocinolone acetonide, as well as combination or solo therapy with focal laser or micro-pulse laser, or both.
NO SOLO ACTS
Do not rely solely on macular OCT when examining patients with diabetes, especially in those patients who have long-standing disease and/or poor glycemic control. Such patients occasionally present with excellent visual acuity and without OCT-based evidence of diabetic macular edema. However, some patients may have proliferative diabetic retinopathy with peripheral neovascularization that the physician can be miss unless they are given a complete exam with dilation, and examination of the peripheral retina, combined with fluorescein angiography — preferably with widefield or ultra-wide fluorescein angiography (Figure 7).

Figure 7. Top: OCT of patient with proliferative diabetic retinopathy without diabetic macular edema. Bottom: Ultra-widefield fluorescein angiography of same patient revealing proliferative neovascularization.
When to obtain an OCT — and why
OCT images are routinely taken to evaluate:
• Vitreous retinal interface anomalies
• Macular traction
• Macular holes
• Macular membranes and puckers
• Macular edema
• Age-related macular degeneration
• Choroidal neovascularization
• Pigment epithelial detachment and drusen
• Central serous chorioretinopathy
• Nonproliferative and proliferative diabetic retinopathy
OCT images of some common retinal diseases are shown in Figures 2, 3 and 4.

Figure 2. Spectral-domain OCT of a full-thickness macular hole.

Figure 3. SD-OCT of a central serous chorioretinopathy.

Figure 4. SD-OCT of choroidal neovascularization secondary to age-related macular degeneration.
In these patients with proliferative diabetic retinopathy, panretinal photocoagulation therapy is recommended and close follow-up with a retinal specialist is essential.
AGE-RELATED MACULAR DEGENERATION
Patients age 50 or older who present with symptoms of distortion or blurred vision require careful examination, as these complaints may be due to macular pathology secondary to age-related macular degeneration. AMD is a leading cause of blindness among Americans who are 65 years or older. OCT is vital in helping to identify drusen, geographic atrophy and ellipsoid zone disruption, indicating a possible choroidal neovascular membrane, as no other imaging device is capable of this (Figure 8).

Figure 8. Left: OCT of drusen. Right: OCT of subretinal fibrosis and subretinal fluid secondary to exudative age-related macular degeneration.
Patients whose eyes have symptoms of macular degeneration need additional imaging tests, such as fundus autofluorescence, to better highlight drusenoid change and geographic atrophy; they also need fluorescein and impedance cardiography (ICG) angiography to identify abnormalities of the blood-retinal barriers.
Earlier referral to a retinal specialist for AMD management may be helpful as the treatment regimen can be complex. To treat wet AMD, the surgeon must decide on whether to initiate anti-VEGF injections, as patient selection is crucial concerning which anti-VEGF agent is optimal, and then deciding on the interval of anti-VEGF injections. Moreover, the complexity of treating AMD lies in the pathophysiology of the breakdown of the blood-retinal barrier and the pharmacodynamics of available treatments. The result is that treatment of AMD is a continuously dynamic process: It requires close patient follow-up; often necessitates changing the type of anti-VEGF agent used; and altering the interval of injection therapy.
MACULAR HOLE
The optical cross section of the retina that OCT provides makes this technology invaluable in helping to differentiate a pseudo-hole from a true macular hole (Figure 9).

Figure 9. Left: Lamellar macular hole. Right: Full-thickness macular hole.
You can observe patients with a lamellar macular hole, as the natural history of lamellar macular holes trend towards long-term stable visual acuity, generally without requiring surgical intervention. However, refer a full-thickness macular hole to a retinal specialist for management, as they frequently need surgical intervention for macular hole closure with pars plana vitrectomy and membrane peel, if medical therapy fails. The chronicity and size of a full-thickness macular hole can influence surgical success of macular hole closure rates, thus these patients benefit from early referral to a retinal specialist.
BENEFITS NOW AND LATER
In conclusion, the use of OCT has facilitated diagnosis and treatment of retinal diseases for comprehensive ophthalmologists as well as retina specialists. Despite the advances in OCT imaging, it is important not to forget the value of other imaging modalities, as OCT may often provide only one piece of the puzzle in complex cases that can benefit from more comprehensive work-up with fundus autofluorescence, fluorescein and ICG angiography and electroretinogram.
Most recently, developments in OCT angiography have shown promise in expanding the utility of OCT for vascular diagnosis in the macula. OCT angiography enables noninvasive visualization of blood flow in the retina and choroid capillary network. This visualization can help identify neovascularization or ischemia of the retina (Figure 10).

Figure 10. Left: OCT angiography revealing capillary non-perfusion despite right, normal appearing fundus image.
The clinical applications of OCT angiography are expansive. They will be great adjunctive tools to fluorescein angiography in helping identify functional retinal vascular pathology noninvasively through routine monitoring. OM
REFERENCES
1. Huang D, Swanson EA, Lin CP, Schuman JS, et.al. Optical coherence tomography. Science. 1991;254:1178-1181.
2. Bélair ML, Kim SJ, Thorne JE, Dunn JP, Kedhar SR, Brown DM. Incidence of cystoid macular edema after cataract surgery in patients with and without uveitis using optical coherence tomography. Am J Ophthalmol. 2009;148: 128-135.
3. Wessel MM, Aaker GD, Parlitsis G, Cho M, D’Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32:785-791.
4. Bottoni F, Diero AP, Giani A Orini C, et al. The natural history of lamellar macular holes: A spectral domain optical coherence tomography study. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2013; 251:467-475.
5. Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45-50.
All images featured in this article are courtesy of Jessica G. Lee, MD and the Photography Department at New York Eye and Ear Infirmary of Mount Sinai.
About the Authors | |

| Jessica Lee, MD, is vitreoretinal surgery fellow at New York Eye and Ear Infirmary of Mount Sinai. Contact her at: jlee@nyee.edu. She has no financial relationships to disclose. |

| Richard B. Rosen, MD, is director, ophthalmology research, and professor of ophthalmology at Mount Sinai School of Medicine, and surgeon director and chief, retina service at N.Y. Eye and Ear infirmary of Mount Sinai. Dr. Rosen has financial relationships with Optovue, Allergan, Quantel, Clarity, Opticology, Ocata, Nano Retina and Regeneron. |








