Provide fast, targeted relief with the latest diagnostic tools and novel therapies.
Emerging Concepts in Dry Eye Patient Management
Dr. Sheppard: Today, we’ll discuss how our management of keratoconjunctivitis sicca, a.k.a. ocular surface disease or dry eye, has evolved. Not only do we have new knowledge, with peer-reviewed scientific backing1 but we also have access to new diagnostic tools and therapies to target specific etiologies. All of our panelists have a special interest in this topic, so I would like to find out what trends you’re seeing in your dry eye patient population.
Dr. Jackson: Every day, I’m challenged when I see patients who have ocular surface disease. They may have aqueous deficiency problems, evaporative dry eye, ocular allergy or a combination of several conditions causing or exacerbating their dry eye. Often, these patients have concomitant diseases, such as diabetes or thyroid disease, and they may be taking medications that also exacerbate their dry eye. These factors, combined with the effects of aging, present a complicated picture. I must make a diagnosis quickly, and most of the time, I must educate or re-educate the patient about proper lubricating agents and other therapies, including medications and nutritional supplements.
“Ocular surface disease is a complex and kinetic disease. Every patient has a different manifestation of the disease, and no single test leads to the diagnosis. That’s why, in my practice, we put a great deal of energy into creating an ocular surface signature for individual patients.”
— Victor L. Perez, MD
Dr. Perez: Ocular surface disease is a complex and kinetic disease. Every patient has a different manifestation of the disease, and no single test leads to the diagnosis. That is why, in my practice, we put a great deal of energy into creating an ocular surface signature for individual patients. We have standardized how we perform all of our tests and measurements, including corneal staining, tear breakup time, lid margin evaluation, imaging with ultrahigh resolution OCT and tear osmolarity measurement. By developing a signature for these patients, we can start a new form of therapy, if necessary, and standardize the therapy according to each patient’s needs.
Dr. Sheppard: Do patients today have a better awareness of their condition and what they can do to control it compared with patients 5 or 10 years ago?
Dr. Donaldson: I believe they do. Often, by the time patients reach us at Bascom Palmer, they’ve seen several other eyecare specialists. They come to us with some type of diagnosis, whether it is dry eye, meibomian gland dysfunction or a conglomerate of ocular surface diseases. They have some knowledge, but I have to educate them further. In fact, I often go over certain aspects of your dry eye and nutrition study,1 Dr. Sheppard, because many of my patients are educated and looking for second or third opinions. After years of difficult symptoms and ineffective treatment with over-the-counter artificial tears, they are frustrated. They need to advance to the next level of therapy, and education is important.
Dr. Lemp: When I look at the state of our knowledge a decade or two ago, I’m appalled at how little we knew. What’s more, we didn’t know how little we knew. Two things have happened. One, we now have much more data, giving us better insight into the pathophysiologic mechanisms of dry eye. Two, our patients are much more sophisticated, largely because of consumer advertising. People know the term “dry eye.” They know it’s a problem, and they’re seeking answers. They usually have an idea of what’s wrong before they come to see us.
Dr. Sheppard: Dr. Perez, in your practice, do patients accept one form of treatment more readily than another?
Dr. Perez: Yes and no. The “yes” is the patient with inflammatory disease associated with a connective tissue disorder, such as Sjögren’s syndrome, graft-versus-host disease or rheumatoid arthritis. We have an approved eye drop to treat inflammation, so therapy is straight-forward for those patients. The “no” patients are those who have a mixed diagnosis. That’s a Pandora’s box that we need to open, so we can tell patients what they have and how we’ll treat it.
DIAGNOSTIC ADVANCES
Dr. Sheppard: What tests do you use to confirm a diagnosis of dry eye or mixed mechanism disease with meibomitis?
Dr. Jackson: I have a comprehensive ocular surface practice, so our testing runs the gamut: corneal staining, conjunctival staining with lissamine green and tear breakup time. I also use devices, such as the LipiView ocular surface interferometer (TearScience) to look at the meibomian oil layer, and the TearLab Osmolarity Test (TearLab Corporation). With data from all of these tests and based on the latest definition of dry eye that includes inflammation and elevated osmolarity,2 I classify the disease, using the International Task Force (ITF) on Dry Eye classification system.3
Dr. Sheppard: Dr. Donaldson, what test do you use to differentiate between evaporative and meibomian disease? What guides you in your selection of treatments?
Dr. Donaldson: In my practice, we perform the TearLab Osmolarity Test on all dry eye patients. In my opinion, it is more reproducible and more correlated with the severity of dry eye than any other test. Of course, despite the luxury of having rapidly advancing technology to assist us in the diagnosis and treatment of these challenging patients, nothing can replace a detailed history and slit lamp examination with careful attention to the ocular surface and lid margin. For those patients in whom symptoms seem out of proportion to physical signs on examination, I find the TearLab Osmolarity Test particularly useful.
RAPID RESOLUTION
Dr. Sheppard: Often, we see patients who are in a blazing hurry to have LASIK or cataract surgery. If they have dry eyes, what approach do you recommend?
Dr. Lemp: I think we’d all agree that to achieve optimum outcomes in refractive or cataract surgery requires a normal ocular surface. Surgeons need a way to determine which patients may have a high probability of a poor result. I’ve been involved in the development of the TearLab Osmolarity Test, and I think we have some convincing data, which shows that the test is a good way to identify people who are at risk.4 Once the diagnosis is confirmed, I would start a treatment regimen to stabilize the corneal surface.
Dr. Sheppard: For fast results, would you use a steroid?
Dr. Jackson: Many treatments, such as topical cyclosporine, can take 6 to 8 weeks to have maximal effect, so I use a topical steroid to help patients feel better immediately. Patients have to know they’re getting better to be motivated to continue treatment, and that’s where tear osmolarity measurement is helpful. It’s a global marker. After learning the baseline measurements, a patient becomes interested in improving that reading, which motivates him to adhere to therapy.
Dr. Sheppard: Dr. Perez, are you an advocate of topical steroids or Restasis (cyclosporine ophthalmic emulsion, 0.05%; Allergan Inc.). What is your preferred therapy?
Dr. Perez: I see many patients referred by rheumatologists, and those patients have autoimmune components. For them, I think a steroid should be a first-line therapy.
Supplement Shown to Benefit Dry Eye, Relieve Symptoms
A randomized, placebo-controlled, double-masked clinical trial led by John D. Sheppard, MD, MMSc* and Stephen C. Pflugfelder, MD**, evaluated the effects of a dietary supplement (HydroEye®, ScienceBased Health®) on moderate-to-severe dry eye in postmenopausal women.1 The trial was conducted at Virginia Eye Consultants and Baylor College of Medicine.
Methods: Participants were randomly assigned to receive two softgels twice daily of the supplement or placebo for 24 weeks. The supplement consisted of black currant seed and fish oils (sources of GLA/ALA and EPA/DHA, respectively), antioxidants and nutrient co-factors.
Efficacy outcomes, assessed at baseline and at 4, 12 and 24 weeks, included Ocular Surface Disease Index (OSDI), Schirmer’s test, TBUT, conjunctival fluorescein and lissamine green staining and corneal topographic indexes. Conjunctival impression cytologies were obtained and immuno-stained for inflammatory biomarkers.
“The data from the HydroEye clinical trial is impressive. People may not realize just how difficult it is to obtain significant findings in clinical trials with dry eye. You have to have a pretty potent effect to overcome the variability that’s inherent in these kinds of trials.”
— Michael Lemp, MD
Symptoms: Mean OSDI scores progressively improved with supplement use, achieving significance at 12 weeks and 24 weeks (P = 0.004), and were significantly improved vs. placebo at 24 weeks (P = 0.05) (Figure 1).
Inflammation: Inflammation was assessed by two inflammatory biomarkers, HLA-DR and CD11c (Figures 2-3). CD11c is a molecule on certain dendritic cells that regulates their migration. HLA-DR, a dendritic cell activation marker, increases upon exposure to inflammatory cytokines. Increased conjunctival HLA-DR expression has previously been observed in dry eye.2
Inflammation did not progress in the supplement group, while it continued to progress in placebo. In the placebo group, CD11c intensity was significantly elevated at 12 and 24 weeks (P= 0.004) and HLA-DR intensity was significantly higher at 24 weeks (P=0.03). At study’s end, the placebo group had significantly increased intensity for both biomarkers (P= 0.001) compared to the supplement group.
Corneal Smoothness: Corneal surface irregularity can contribute to ocular irritation and impact the quality of vision. Two corneal topographic regularity indices (surface regularity index [SRI] and surface asymmetry index [SAI]) — both previously reported to increase in dry eye3 — were assessed as measures of corneal smoothness. SAI remained unchanged with supplement treatment, but increased in placebo users, reaching significance between groups at 24 weeks (P=0.005). No between-group differences were seen for SRI.
Summary of Findings
Supplement treatment significantly improved dry eye symptoms. The supplement group experienced no progression of ocular surface inflammation, while inflammation worsened in placebo-takers. Corneal smoothness was also maintained with supplement use, while surface irregularity progressed in placebo takers.

Figure 1 OSDI scores (mean ± SEM) decreased consistently over the 24-week treatment period after supplement treatment.
†Significant improvement when compared to baseline, P = 0.004.
*Significant improvement compared to placebo, P = 0.05.

Figure 2 Fluorescence intensity (mean ± SEM) of HLA-DR-positive dendritic cells in conjunctival impression cytology.
†Significant increase in HLA-DR-positive cell staining compared to baseline, P = 0.03.
*Significantly greater staining intensity compared to supplement, P = 0.001.

Figure 3 Fluorescence intensity (mean ± SEM) of CD11c- positive dendritic cells in conjunctival impression cytology.
†Significant increase in CD11c-positive cell staining compared to baseline, P = 0.004.
*Significantly greater staining intensity compared to supplement, P = 0.001.
*Virginia Eye Consultants and Eastern Virginia Medical School
** Cullen Eye Institute, Baylor College of Medicine
REFERENCES
1. Sheppard JD Jr, Singh R, McClellan AJ, Weikert MP, Scoper SV, Joly TJ, Whitley WO, Kakkar E, Pflugfelder SC. Long-term supplementation with n-6 and n-3 PUFAs improves moderate-to-severe keratoconjunctivitis sicca: A randomized double-blind clinical trial. Cornea 2013;32:1297-1304.
2. Pisella PJ, Brignole F, Debbasch C, et al. Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca. Ophthalmology. 2000;107:1841–1849.
3. de Paiva CS, Lindsey JL, Pflugfelder SC. Assessing the severity of keratitis sicca with videokeratoscopic indices. Ophthalmology. 2003; 110:1102–1109.
The Anti-inflammatory Omega: GLA
While omega-3 fatty acids from fish oil are sometimes used as a standalone treatment for dry eye, only the unique fatty acid, GLA (gamma-linolenic acid) has a body of clinical evidence supporting its effectiveness in dry eye patients. GLA has been shown in seven published controlled trials to significantly improve dry eye signs and/or symptoms in early to moderate dry eye, post-PRK surgery, meibomian gland dysfunction, Sjögren’s Syndrome, postmenopausal women, tear-deficiency and contact lens wear.1-7
GLA, found in just a few plant-based oils from the seeds of black currant, evening primrose and borage, is not present in fish or flaxseed oils and cannot be obtained in meaningful levels from the diet.8
Research on the role of fish oil omega-3s administered alone is limited for ocular surface disease; only one randomized controlled clinical trial to date has shown significant dry eye benefit from fish oil thus far.9
Unlike the pro-inflammatory omega-6 fatty acids that are abundant in the Western diet, GLA is a precursor of anti-inflammatory prostaglandin E1.10 Thus GLA has a potent anti-inflammatory effect, especially when combined in a balanced ratio with fish oil. Administering GLA with EPA/DHA has been clinically shown to potentiate the activity of GLA by decreasing production of pro-inflammatory arachidonic acid and prostaglandin E2.11,12 Co-supplementation of these fatty acids has also been reported to reduce levels of inflammatory biomarkers (HLA-DR and CD11c) in the conjunctival epithelium of dry eye patients.1,13
REFERENCES
1. Sheppard JD Jr, Singh R, McClellan AJ, Weikert MP, Scoper SV, Joly TJ, Whitley WO, Kakkar E, Pflugfelder SC. Long-term supplementation with n-6 and n-3 PUFAs improves moderate-to-severe keratoconjunctivitis sicca: A randomized double-blind clinical trial. Cornea 2013;32:1297-1304.
2. Kokke KH, Morris JA, Lawrenson JG. Oral omega-6 essential fatty acid treatment in contact lens associated dry eye. Cont Lens Anterior Eye. 2008;31:141-146.
3. Pinna A, Piccinini P, Carta F. Effect of oral linoleic and gamma-linolenic acid on meibomian gland dysfunction. Cornea. 2007;26:260-264.
4. Aragona P, Bucolo C, Spinella R, et al. Systemic omega-6 essential fatty acid treatment and pge1 tear content in Sjögren’s syndrome patients. Invest Ophthalmol Vis Sci. 2005;46:4474-4479.
5. Barabino S, Rolando M, Camicione P, et al. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003;22:97-101.
6. Macri A, Giuffrida S, Amico V, Iester M & Traverso CE. Effect of linoleic acid and gamma-linolenic acid on tear production, tear clearance and on the ocular surface after photorefractive keratectomy. Graefes Arch Clin Exp Ophthalmol. 2003;241:561-566.
7. Creuzot-Garcher C et al. Efficacy assessment of Nutrilarm, a per os omega-3 and omega-6 polyunsaturated essential fatty acid dietary formulation versus placebo in patients with bilateral treated moderate dry eye syndrome. J Fr Ophtalmol. 2011;34:448-455.
8. Kapoor R, Huang YS. Gamma linolenic acid: an anti-inflammatory omega-6 fatty acid. Curr Pharm Biotechnol. 2006;7:531–534.
9. Kangari H, Eftekhari MH, Sardari S, Hashemi H, Salamzadeh J, et al. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology, 2013;120:2191-2196.
10. Chilton FH, Rudel LL, Parks JS, Arm JP, Seeds MC. Mechanisms by which botanical lipids affect inflammatory disorders. Am J Clin Nutr 2008; 87(suppl):498S–503S.
11. Barham JB, Edens MB, Fonteh AN, Johnson MM, Easter L, Chilton FH. Addition of eicosapentaenoic acid to gamma-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans. J Nutr. 2000;130:1925–1931.
12. Viau S, Leclère L, Buteau B, Grégoire S, Acar N, et al. Polyunsaturated fatty acids induce modification in the lipid composition and the prostaglandin production of the conjunctival epithelium cells. Graefes Arch Clin Exp Ophthalmol. 2012;250:211–222.
13. Brignole-Baudouin F, Baudouin C, Aragona P, Rolando M, et al. A multicentre, double masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmol. 2011;89:e591–e597.
Dr. Sheppard: Is there an acceptable risk profile in topical steroid use?
Dr. Perez: Yes. You have to be responsible. I like to use loteprednol, which has a good safety profile. I prescribe a 4- to 6-week course of therapy, and I make sure patients return for followup, so I can detect any side effects.
NUTRITIONAL THERAPY
Dr. Sheppard: Dr. Donaldson, when treating a patient with ocular surface disease, when do you introduce a nutritional supplement, such as HydroEye (ScienceBased Health)?
Dr. Donaldson: I introduce HydroEye early for almost all of my dry eye patients, whether their disease is mild or moderate to severe. I educate my patients about the benefits of gamma-linolenic acid and omega-3 polyunsaturated fatty acids for treating dry eye. They like the idea that it’s an oral agent that has the potential to help them holistically.
Dr. Sheppard: Are there any contraindications to using this type of nutritional supplement?
Dr. Jackson: One contraindication is for people allergic to fish.
Dr. Sheppard: Certainly contraindications are rare. Occasionally, patients taking anticoagulants such as Warfarin (Coumadin, Bristol-Myers Squibb) may require adjustments to their regimen after 3 to 4 months of use because GLA can have a mild anticoagulant effect. Thus, I always clear HydroEye treatment with the appropriate managing healthcare professional. When do you start HydroEye, Dr. Jackson?
Dr. Jackson: I like to start nutritional supplementation with HydroEye right away, along with any acute therapies I deem necessary, because the treatment effect isn’t immediate. I want to get patients feeling better fast and keep them motivated to stay better. I offer HydroEye as part of a long-term strategy that may include a topical anti-inflammatory that has a low side-effect profile.
Case 1. 34-year-old Hispanic Male | Multiple Environmental Factors
KEY PATIENT DATA
► Traveling executive
► Occasional burning and itching
► Difficulty tolerating contact lenses for more than 4 hours/day
► Low carbohydrate diet: lost 80 pounds
► Using fexofenadine for chronic allergic rhinitis
► Using olopatadine for chronic allergic conjunctivitis
Dr. Sheppard: This 34-year-old man, a traveling executive, has occasional ocular burning and itching . He has difficulty tolerating contact lenses more than 4 hours a day, but he feels wearing them is essential to his occupation. He takes olopatadine for chronic allergic conjunctivitis and fexofenadine for chronic allergic rhinitis. He recently lost 80 pounds rapidly on a low carbohydrate diet. He has a diffuse, confluent inferior punctate keratopathy pattern in a classic meibomian gland distribution. Dr. Donaldson, what are your impressions of this patient?
Dr. Donaldson: As someone who travels for work, this patient is often subjected to the dry environment of an airplane; and living in the Miami area, he’s exposed to air conditioning frequently. He probably sits in front of a computer all day and has a decreased blink rate. All of these factors contribute to dryness and can take a toll on the ocular surface. His occasional burning and itching may or may not be associated with contact lens wear. In addition, he’s taking an antihistamine, which is drying. I would hope he’s already using artificial tears.
Dr. Sheppard: How would you treat this patient?
Dr. Donaldson: With his advanced meibomian gland dysfunction and his evaporative dry eye, this patient is a candidate for lid hygiene, and I think he’s a great candidate for treatment with LipiFlow, as well. Sometimes it’s better to start one therapy at a time and evaluate its effect before adding a therapy. Certainly, I would start nutritional supplementation and lid hygiene right away. I’d bring him back and offer him LipiFlow. I think he would do well with that intervention.
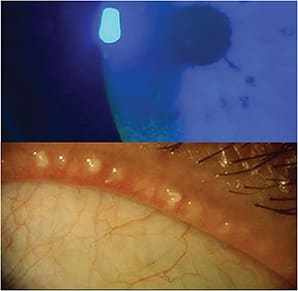
Dr. Perez: When patients have a complaint of itching, we have to determine if it’s from allergy or from ocular surface disease. Another consideration is demodex. When a patient doesn’t respond to therapy, look for telltale accumulations on the lashes.
Dr. Sheppard: For patients with allergies who need a systemic antihistamine, I’m a proponent of oral montelukast to mitigate their symptoms without causing dryness. Another option is to switch from a topical antihistamine to a low dose of a topical steroid, which reduces the dryness and increases comfort.
Dr. Jackson: If you must prescribe a topical antihistamine such as bepotastine besilate 1.5% (Bepreve, Bausch + Lomb), you should use one that’s more H1 receptor-specific to minimize dry eye side effects.
Dr. Donaldson: In reviewing this case, I see the patient recently lost 80 pounds. Many of the post-bariatric surgery patients I’ve seen receive nutritional counseling for the first year after surgery, but after that, they often stop taking their supplements and become deficient in vitamin A. When they add a vitamin A supplement to their regimen, their ocular surface often heals. I’ve been impressed by that.
New Diagnostic Tool for Sjögren’s Syndrome
Dr. Sheppard: Sjögren’s Syndrome is an important, frequently unrecognized autoimmune condition strongly associated with dry eye, xerostomia, and a surprisingly morbid collection of systemic complications including vasculitis and interstitial pneumonitis.
Dr. Jackson: The FDA recently approved a panel of diagnostic tests that have high sensitivity and specificity for an earlier detection of Sjögren’s syndrome (Sjö; Nicox Inc./Immco Diagnostics Inc.). Eyecare practitioners can share results from this finger-prick test or standard laboratory venipuncture with primary care physicians and rheumatologists.
Dr. Lemp: That’s an important point, because it’s often the eyecare practitioner who first identifies a patient with Sjögren’s syndrome, and if we miss it, it may go undetected and untreated for many years. With this new, proactive test, we can identify these patients before significant pathology develops.
Case 2. 70-year-old Asian Female | Cataract and Glaucoma
KEY PATIENT DATA
► 20/50 cataracts
► 20/100 glare
► IOP 22 OU
► CCT .525 OD, .530 OS
► Topical timolol and brimonidine
► Early visual field changes
► Normal OCT, C/D ratio: 0.6 OU
Dr. Sheppard: This 70-year-old woman has 20/50 cataracts, 20/100 glare, IOP of 22 mmHg OU, with somewhat thin central corneal thickness, early visual field changes, a normal OCT and borderline cup-to-disc ratio. She is using topical timolol and brimonidine. This patient clearly has a glaucoma-compromised ocular surface, with a chronic, diffuse, punctate keratopathy, conjunctival injection, telangiectasia and some architectural damage to the meibomian orifices. Would you operate on this patient tomorrow, Dr. Jackson?
Dr. Jackson: I wouldn’t operate on this patient tomorrow. This is a classic case of medication toxicity, which isn’t unusual with glaucoma agents. I would take a step-wise approach, stopping the glaucoma medications, starting nutritional therapy and treating the ocular surface. If a steroid is indicated, I would prescribe Lotemax Gel (loteprednol etabonate ophthalmic gel, Bausch + Lomb) 0.5%, which is more IOP-friendly than other agents. Although this patient doesn’t have a severe meibomian gland problem, I would recommend lid maintenance. I would routinely monitor her condition using the TearLab Osmolarity test, which is usually reimbursable for patients using glaucoma medications.

Dr. Sheppard: Some glaucoma agents are more deleterious to tear production than others, and I think the beta-blockers are at the top of this list. You might consider eliminating the beta-blocker preoperatively by performing selective laser trabeculoplasty. I’m a strong proponent of concomitant microincisional glaucoma surgery (MIGS) with cataract surgery, using either the Trabectome (NeoMedix Corporation) or iStent implantation (Glaukos Corporation).
Dr. Jackson: Another option is to prescribe a preservative-free glaucoma drop such as tafluprost (Zioptan, Merck) and/or dorzolamide/timolol temporarily until the ocular surface heals.
Dr. Perez: I’ve found that some patients with medicamentosa respond well to autologous serum tears.
Dr. Sheppard: You use autologous serum rather early in your protocol. Are you confident that you have an excellent pharmacy to prepare it for you?
Dr. Perez: We make the solution ourselves at Bascom Palmer, and I monitor the patients closely. Our patients have tried everything, so this gives us another therapy that may be beneficial.
Dr. Donaldson: Serum tears have been incredibly beneficial for my patients, as well. They say it’s been like a miracle in their lives.
Dr. Sheppard: You are indeed fortunate to have such a reliable pharmacy in house. Topical preservative free recombinant human immunoglobulin tears, bypassing the preparation challenges of serum tears, are currently in Phase II clinical trials (R-tech).
New Entity in the Dry Eye Spectrum
Dr. Sheppard: At the other end of the spectrum from patients with moderate to severe dry eye are those in the early stage of the disease, many of whom have no corneal staining. Researchers in Japan recently published a paper, describing what they believe is a new entity: short tear film breakup time (BUT) dry eye.1
They evaluated 77 patients who had a rapid breakup of their tear film but no staining. They found that mainly young men and menopausal women contracted short BUT-type dry eye. They concluded that changes in sex hormones, work at the computer and contact lens wear may be causative factors, and they found the disease could not be controlled by artificial tears and punctal plugs alone.
So staining is important when it’s there, but when it’s not there, it doesn’t rule out the fact that some ocular surface problems might be developing.
Case 3. 34-year-old African American Male | Post-LASIK
KEY PATIENT DATA
► Myopic LASIK 2 years ago OU
► Constant burning and dryness
► Difficulty working, driving and reading
► Osmolarity 325/355
► TBUT < 3 seconds OU
► Severe central SPK
Dr. Sheppard: This 34-year-old man had bilateral LASIK surgery 2 years ago to correct myopia. He has constant burning and dryness and difficulty working, driving and reading. His tear osmolarity is 325 mOsms/L and 355 mOsms/L. His tear breakup time is less than 3 seconds, and he has severe central superficial punctate keratopathy (SPK). Dr. Lemp, what is your impression of this individual, who was perhaps ill advised to have LASIK?
Dr. Lemp: This patient has a hyper-concentrated tear film and evidence of ocular surface damage in the visual axis, which is compromising his visual acuity. Post-LASIK neuropathy, rather than changes in tear secretion, is likely playing a significant role. In addition, an osmolarity of 355 mOsm/L indicates that this is a severe dry eye condition. The osmolarity difference between both eyes is 30 mOsm/L, indicating a loss of homeostatic control and the significant instability of the tear film is a hallmark of dry eye disease.
Dr. Sheppard: How would you proceed with his treatment?
Dr. Lemp: First, I would prescribe a regimen to heal the central SPK. We tend to overlook artificial tears, but there are some very good products that stabilize the tear film and drive down osmolarity significantly. I would also start HydroEye for this patient. We don’t have much information about his lid margins, and he’s young to have meibomian gland dysfunction, but it’s something I would explore. He might be experiencing changes because of his occupation, perhaps spending more time staring at a computer and not blinking. I would start a stepwise program, adding the appropriate therapies as we’ve discussed.
Dr. Jackson: I find osmolarity data incredibly useful when I’m treating post-LASIK patients, especially those who come to me after seeing numerous other providers. Abnormal osmolarity readings will confirm that the cornea is truly compromised and that, despite any discrepancy in signs and symptoms, the patient needs aggressive, systematic, intelligent intervention. I’ve found that even patients who have tried multiple therapies, all of which have failed or were incompletely prescribed or taken, will immediately adapt to an aggressive nutritional intervention. Sometimes, that has a remarkable positive effect in this unusual but underserved population.
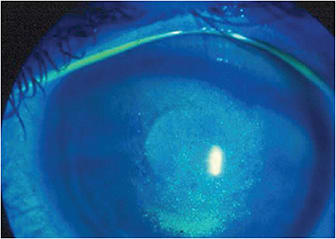
Dr. Lemp: Patients like to have an anchor to which they can relate. They are accustomed to doing that with other health issues, such as blood pressure, cholesterol and blood sugar. They understand the goal is to bring their readings into normal range, and they work with you to achieve this goal.
Dr. Donaldson: Many dry eye treatments take several months to take effect. Would you use punctal plugs in this patient to provide fast relief?
Dr. Lemp: Punctal plugs can be quite useful in certain patients with dry eye. In fact, I’m currently involved in a study of the effect of punctal plugs on tear osmolarity. In general, tear osmolarity tends to decrease when the puncta are plugged. Keep in mind, however, that punctal plugs are contraindicated in people who have clear evidence of inflammation on the corneal surface. Plugs interfere with drainage, causing the corneal surface to be bathed in fluid that is toxic to it. You must choose your patients carefully, but for most people who have dry eyes, I believe punctal plugs can play an important role.
Dr. Jackson: Dr. Donaldson’s point about the time it takes for some therapies to take effect is right on, because depending on the urgency and severity of a case, I may be more willing to move plugs to an earlier position in my algorithm. Otherwise, I’m following the basic playbook, which tells us to normalize the inflammation and then insert the plugs. In a desperate case like this one, however, I might decide to put in the plugs now and make the patient functional tomorrow.
Dr. Donaldson: In addition, starting a therapy that provides quick relief often encourages patients to adhere to their long-term therapies.
Dr. Sheppard: Dr. Jackson, do you have a standard preoperative protocol for your LASIK patients?
Dr. Jackson: Patients who want LASIK tend to be young and healthy, and generally, they take care of themselves. I always start them on a nutritional supplement and Restasis the day they stop wearing their contact lenses, which is the day they’re committing to their overall outcome.
Dr. Donaldson: Do you do that with all of your LASIK patients or just higher risk patients?
Dr. Jackson: All of my LASIK patients follow that regimen the day they stop wearing their contact lenses. If LASIK neurotrophic epitheliopathy is a problem postoperatively and they’re already using a steroid and an antibiotic, I add punctal plugs. I know patients will get better, and adding the plugs helps them get through the symptoms faster. I use tear osmolarity to guide the treatment process.
Case 4. 80 year-old White Female | Autoimmune-associated Dry Eye
KEY PATIENT DATA
► BCVA 20/30 OU
► Glare 20/50 OU
► Mild nuclear sclerosis
► Cannot read or drive
► SPEED™ questionnaire: 14/28
► OSDI: 33/48
► Xerostomia with gingivitis
Dr. Sheppard: This 80-year-old woman has best-corrected visual acuity of 20/30 OU with glare of 20/50 OU and mild nuclear sclerosis. She cannot read or drive. Her Standard Patient Evaluation of Eye Dryness (SPEED)7 questionnaire score has increased from 14 to 28, and her ocular surface disease index (OSDI) score is 33 of 48. She has xerostomia and gingivitis. I believe it’s somewhat urgent to operate on her. Dr. Perez, how would you proceed?
Dr. Perez: The first thing to do is what I tell my fellows and residents: Put away the slit lamp, turn on the room light and talk to the patient. Get a good review of systems, and find out what medications she is taking. Examine her hands for signs of rheumatoid arthritis. Because she has dry mouth and gingival inflammation, we need to consider inflammatory diseases, such as Sjögren’s syndrome, that might cause dry eye. I would carefully examine the conjunctival surface for cicatrizing changes to rule out ocular cicatricial pemphigoid, which could be causing this form of dry eye. Once you rule out what you need to treat systemically, then you can focus on the eye.

Dr. Sheppard: How would you treat this patient?
Dr. Perez: With a diagnosis of autoimmune-associated dry eye, I would quickly start anti-inflammatory therapy. My preferred treatment is loteprednol twice a day along with a 4- to 6-week course of Restasis.
This patient also has filaments, which need to be debrided and cleaned. If this is an evolving and severe filamentary keratopathy, I would then follow an aggressive step ladder approach to prevent surface damage and alleviate the severe ocular pain associated with this condition. I like to use bandage contact lenses for these patients to provide some comfort until I can stabilize the ocular surface. These patients must be followed closely, because if this is an autoimmune-mediated dry eye, the cornea can melt rapidly.
Dr. Sheppard: As this case illustrates, a significant majority of elderly Americans are malnourished. They may be cooking for themselves without attention to a balanced diet. They may be dehydrated, too. In addition, one of the number one diagnoses in people over the age of 80 is depression, and that’s concomitant with poor self-care habits. We tend to forget these subtleties that are central to the primary care of geriatric patients.
Dr. Jackson: For this patient, I’d also use punctal plugs initially. Once filaments have formed, the ITF classification is 3, and the recommendation is to start an anti-inflammatory agent, use punctal plugs and start a nutritional supplement.
Early Warning Sign: Intereye Variability in Osmolarity
Dr. Jackson: I understand intereye variability in tear osmolarity can be an indicator of early-stage dry eye. Many of us just look at the absolute number for each eye, but a difference in osmolarity between the eyes could be a hallmark of early disease. Would you agree, Dr. Lemp?
Dr. Lemp: Absolutely. The acceptable intereye variability, which is built into the technology of the TearLab Osmolarity Test, is about 8 mOsms/L. Even if you have two readings that aren’t above 308 mOsms/L, a difference of 12 mOsms/L or 13 mOsms/L between eyes may be an indication of tear film instability and dysfunction. Remember that the normal range has about 25 mOsms/L in it, and baseline for some people may be in the low part of that range, while others have a high thermostat, if you will. When you start to see differences in osmolarity of > 8 mOsms/L between the left and right eye, however, you’re seeing a breakdown in the homeostatic control and the beginning of instability coming into that system.1 So, intereye variability can be an early warning sign.
Dr. Sheppard: I adhere to several guidelines in my practice: For example, I treat everyone as if they were my mom or dad. Every patient who needs cataract surgery is a potential candidate for premium IOLs, and everyone with ocular surface disease should be offered a nutritional supplement. We need to be proactive in every aspect of ophthalmic care, and nutritional supplementation is one way to do it safely.
Dr. Perez: I’m intrigued and excited by what I’m learning from this panel about using a nutritional supplement as a first-line therapy for patients with inflammatory diseases of the eye secondary to autoimmune disorders. After talking to the scientific panel from ScienceBased Health, I’ve been impressed with how lipids can be manipulated to stimulate anti-inflammatory cascades. I’m going to start using nutrtional supplements as one of my first lines of therapy.
Dr. Sheppard: I look upon nutritional supplementation as the anchor therapy for meibomian gland disease, as well. On day one, whether a patient has a dry eye or meibomian gland dysfunction diagnosis, I start him on a nutritional supplement.
THERMAL PULSATION
Dr. Sheppard: Do you see a major role for thermal pulsation to treat patients who have mixed mechanism dry eye?
Dr. Lemp: Definitely. Our current understanding of dry eye is that there are two mechanisms: aqueous tear deficiency, when the lacrimal glands don’t produce enough tears, and excessive evaporative loss, which is commonly caused by meibomian gland dysfunction. Among the people with meibomian gland dysfunction, a significant number have blocked glands. Until now, we’ve had few effective treatments. We know that patients are not highly compliant when we advise them to use warm compresses, and in fact, warm compresses, which are applied to the anterior lids, aren’t particularly effective.
Dr. Sheppard: What is different then about thermal pulsation with the LipiFlow device?
Dr. Lemp: The LipiFlow device applies warmth to the upper and lower eyelids while also gently massaging the lids. The pulsatile aspect actually breaks up the blockages to restore lipid secretions. The engineering that went into developing this and the results I’ve seen from it are impressive.
Dr. Perez: I’ve been using the LipiFlow for several months. The International Workshop on Meibomian Gland Dysfunction5 helped us understand the pathophysiology of this disease. So expressing the glands makes sense. I believe dry eye therapy will be multifaceted, with medical therapy combined with what can be called physical therapy, and thermal pulsation is part of that physical therapy.
Dr. Donaldson: Some of my patients were diagnosed with dry eye by other practitioners, but few paid attention to the lid margin. A patient may have seen three or four doctors who never mentioned lid hygiene or instituted some form of treatment, even if only warm compresses. Some practitioners are overlooking that aspect of this problem that can certainly be helped by treatment with the LipiFlow device or manual expression.
Dr. Jackson: What’s important about the LipiFlow is that we’re finally treating the cause of the problem. When we treat the cause of a problem, it’s usually going to resolve. We haven’t done that in the past, because we didn’t have enough information.
Dr. Sheppard: Dr. Jackson, how do you educate your patients about their treatment regimen, particularly when using the LipiFlow?
Dr. Jackson: I emphasize to patients that they have a chronic disease that waxes and wanes, and even though I’m treating it now, they may need a repeat treatment in the future. It’s not one treatment and done. We need a long-term strategy.
Dr. Sheppard: We pre-treat every LipiFlow patient with HydroEye, then insist upon continuous maintenance therapy. The improved quality of meibomian secretions on polyunsaturated fatty acid therapy facilitates the LipiFlow procedure and likely extends the symptom-free period between treatments.6
PREOPERATIVE PRECAUTIONS
Dr. Sheppard: Surgeons now understand that they need to normalize the ocular surface in terms of dry eye to obtain accurate measurements for surgery, and instituting a topical anti-inflammatory makes sense. Is there also a new awareness that perhaps we need to do the same thing with the lid margin prior to surgical intervention?
Dr. Jackson: The Prospective Health Assessment of Cataract Patients Ocular Surface (PHACO) study8 is a prospective, randomized, cohort of patients in the United States, showing that most patients who are about to have cataract surgery are asymptomatic but have clinical signs of dry eye. If we don’t capture appropriate diagnostic data preoperatively and treat the disease, our outcomes will be affected. The PHACO study raised awareness that we must treat dry eye preoperatively.8
Dr. Sheppard: All of my cataract patients take a nutritional supplement and use the surface intervention or the preventative measure that I believe will be most effective to prepare their ocular surface for surgery.
Dr. Jackson: I focus on acute therapy preoperatively, but for chronic, long-term management, I believe patients should be taking a nutritional supplement right from the start.
Dr. Lemp: In some ways, we need to look out of the box. For example, we place a speculum and shine a bright light in a patient’s eye for a considerable period during surgery. Some patients have pain when we place the speculum. We don’t really control lid pain with a topical anesthetic. That pain may be caused by underlying, unidentified, untreated lid disease that’s a key component of the ocular surface prior to surgery.
Dr. Perez: As we collect more data, we’ll learn more about ocular surface disease and develop algorithms to guide us when deciding whom to treat, when to treat and with what to treat. The more data we collect will make the classification of meibomian gland dysfunction much easier.
Dr. Sheppard: As our panelists can attest, there’s no typical dry eye patient. We’ve selected several cases to illustrate the numerous presentations we see in daily practice how we would manage each patient (See Case Studies on pages 9-12).
PREPARE TO INTERVENE
Dr. Sheppard: Quality of care has become a centerpiece for healthcare regulators who focus upon evidencebased medicine. We’re all cautious to have our cataract surgery candidates screened by retina specialists to determine if there’s the slightest epiretinal membrane, loss of fovea reflex or asymmetric thickening on OCT. We should be just as cautious about the condition of the ocular surface. We now have valid quantitative methods to assess then follow our patients with dry eye. We can now reference a prospective, randomized, multicenter, placebo controlled trial clearly demonstrating the clinical and pathophysiologic merits of nutritional therapy. And new, disease specific treatments have become available for evaporative and aqueous deficiency dry eye. If patients aren’t well-served by their current therapies, we must take the role as their ocular surface interventionalists and eradicate or control that condition, using every means available. ■
REFERENCES
1. Sheppard JD Jr, Singh R, McClellan AJ, Weikert MP, Scoper SV, Joly TJ, Whitley WO, Kakkar E, Pflugfelder SC. Long-term supplementation with n-6 and n-3 PUFAs improves moderate-to-severe keratoconjunctivitis sicca: a randomized double-blind clinical trial. Cornea. 2013 July 23.
2. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75-92.
3. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:163-178.
4. Sullivan BD, Crews LA, Sonmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea 2012;31(9):1000-1008.
5. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922-1929.
6. Lane SS, DuBiner HB, Epstein RJ, Ernest PH, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012 Apr;31(4):396-404.
7. Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea. 2013; 32:1204-1210.
8. Trattler W, Goldberg D, Reilly C. Incidence of concomitant cataract and dry eye: prospective health assessment of cataract patients. Presented at: World Cornea Congress; April 8, 2010; Boston, MA.








