Quick Hits
Pharma group Actavis set to acquire Allergan
The $66 billion deal makes Actavis one of the 10 biggest drug makers in the world.
By Bill Kekevian, Senior Associate Editor
When Actavis and Allergan become one company in a few months, the field of ophthalmology is slated to occupy a hefty percentage “of the total combined entity.” So says Allergan’s Chairman of the Board and CEO, David E.I. Pyott, who, along with CEO and President of Actavis Brent Saunders, spoke with Ophthalmology Management staff shortly after the merger announcement, on November 17. Actavis, the Ireland-based pharmaceutical company, has North American products focused on women’s health, urology, gastroenterology and dermatology. Allergan is the manufacturer of several ophthalmic pharmaceuticals as well as Botox. The transaction, which ends months of speculation about the future of Allergan, is anticipated to close in the second quarter of 2015.
“It is my hope that, in the short term, this acquisition has no effect on the ophthalmologist and optometrist customers Allergan currently has,” says Mr. Pyott. “The product innovations will remain.” The companies see the pairing as more of a combination than an acquisition, say both men. The deal makes Actavis one of the 10 biggest drugmakers in the world.
The deal
Actavis acquired Allergan for $129.22 in cash and 0.3683 Actavis shares for each share of Allergan common stock, according to a press release. Based on the closing price of Actavis shares on Nov. 14, the transaction is valued at about $66 billion, or $219 per Allergan share.
The combined company will be led Mr. Saunders. Actavis Executive Chairman Paul Bisaro will remain executive chairman of the board. Also, following the transaction, two members of the Allergan board of directors will be invited to join the Actavis board.
Research and development
According to Mr. Pyott, ophthalmology will comprise about 15% of the new company’s interests. He ranked the specialty among its top priorities.
“The total spent this year [on research and development] across Allergan and Actavis is about $2.2 billion together,” says Mr. Pyott.
“We anticipate that our budget next year will be about $1.7 billion for research and development,” says Mr. Saunders. “If we see that there are other attractive opportunities to invest in research and development, we will spend more money, and if we believe that some of our programs are not reaching the hurdle dates or the success that we had hoped, we’ll stop spending. I strongly believe that research and development is the lifeline of our company, but we need to spend our money responsibly.” That commitment includes Allergan’s DARpin, an anti-VEGF in late-stage clinical development. “My view on DARpin after diligence and looking at it is very bullish. We think that it could really change the paradigm for patients suffering with AMD, could really reduce the need for painful and uncomfortable injections and so we really consider it to be a game changer,” he explains. “But, we have work to do on it. And we’re committed to getting that work done.”
Plans
Mr. Saunders says the new company will offer advantages, in terms of the supply chain and order fulfillment.
“We already have probably one of the premier supply chains in the world. We produce internally about 44 billion units. We contract to manufacture about another 12 to 15 billion units. In terms of degrees of scales, Allergan currently does ... possibly 2 billion units ... We have the ability to reach 40,000 pharmacies, and we can ship to physician’s offices direct, so it’s a really enhanced integration of capabilities.” OM
Jennifer Kirby, senior editor of Optometric Management, contributed additional reporting to this article.
LASIK report mostly positive
Patients are happy with vision, but some drawbacks remain.
By René Luthe, Senior Editor
Preliminary results from the LASIK Quality of Life Collaboration Project (LQOLCP) should gladden the hearts of refractive surgeons everywhere. The multi-year, multi-site study of the safety and efficacy of LASIK confirms that more than 95% of the 574 respondents achieved 20/20 or better binocular vision, and more than 96% reporting being satisfied with their vision three months postop.
Data from phases II and III of the report, PROWL-1 and PROWL-2 (Patient-reported outcomes with LASIK) also provided a more comprehensive portrait of visual symptoms following surgery. Less than 1% of participants experienced “a lot of” difficulty with, or inability to perform, usual activities without corrective lenses because of visual symptoms such as starbursts, glare, ghosting and halos, according to Malvina B. Eydelman, MD, director, Division of Ophthalmic Device Evaluation, FDA.
But the findings were not entirely upbeat. Up to 45% of participants who had no visual symptoms before surgery reported at least one three months after surgery. The most frequently reported symptom was halos, with up to 35% symptom-free participants reporting them at the three-month mark. Additionally, up to 30% of participants with no symptoms of dry eye before surgery reported dry-eye symptoms at three months post-op. “Further analyses are needed to explore other associations with dissatisfaction, and additional longitudinal studies are recommended to explore the factors associated with, and predictors of, poor outcomes,” Dr. Eydelman says.
The LQOLCP is a partnership of the Department of Defense, the National Eye Institute and the FDA.
QUICK BITS
Lacrivera, a division of Stephens Instruments, has received FDA approval for the VeraPlug Punctal Occluder. The VeraPlug is packaged and preloaded on a sterile, disposable inserter/dilator and is available in three sizes.
Columbus ophthalmologist, Alice Epitropoulos, MD, recently signed a licensing agreement with Eye Care and Cure for the manufacturing, marketing and distribution of her EpiGlare Tester product line, a system to document the effect of glare on vision. A video, which can be viewed at https://www.youtube.com/watch?v=IQV8xmtfiko, explains how the test works to test glare.
The FDA has accepted for priority review the supplemental biologics license application for aflibercept (Eylea, Regeneron Pharmaceuticals) for the treatment of diabetic retinopathy in patients with diabetic macular edema, according to a Regeneron news release. The target action date by the FDA is March 30, 2015. The product is currently approved in the United States for the treatment of wet AMD, macular edema following retinal vein occlusion and DME.
Valeant acquires Nicox diagnostics
Valeant Pharmaceuticals International Inc. has acquired the US ophthalmic diagnostics subsidiary Nicox Inc. from Nicox. Under the terms of the transaction, Valeant has acquired most of the Nicox commercial infrastructure in the United States associated with diagnostics. Nicox intends to concentrate its commercial and development resources on ophthalmic therapeutics.
Researchers develop 3D printed facial prosthesis
Patients are scanned to match socket size, skin tone.
By Zack Tertel, Senior Associate Editor
From car parts to musical instruments, 3D printers have created a diverse group of products. That role is about to get more sundry: they are entering the world of facial prosthesis.
University of Miami researchers developed a process that uses facial scanning software to manufacture a 3D printed facial prosthesis, which they say will create an affordable solution for eye cancer patients with hollow sockets following surgery.
“This overall process really opens up a lot of doors in the medical field in general, including ophthalmology, because you have the ability to personalize the treatment to the individual without driving up the cost,” says Landon Grace, PhD, director of Composite Materials Lab at the University of Miami.
Rather than requiring practices to purchase a 3D printer, which can cost up to $100,000, the plan is for practitioners to remotely utilize a topographical scanner, which Dr. Grace says can range from $3,000 to $5,000.
“We want to give people access to this opportunity — anything from centrally located kiosks where people can go to get scanned to locating scanners in individual practices,” Dr. Grace says.

University of Miami researchers developed a 3D printed facial prosthesis to fill an eye cancer patient’s hollow socket after her right eye was removed in surgery.
After a scan, the patient’s facial data are sent to the lab, and a customized prosthesis, which matches the patient’s socket size, shape and skin tone is made. While patients currently pay $10,000 to $15,000 out of pocket for conventional facial prosthesis, which take weeks to produce, a patient’s cost for the 3D printed prosthesis is estimated at $1,000 to $2,000, and it can be shipped back to the patient within a day of receiving the data, says David Tse, MD, professor of ophthalmology at the Bascom Palmer Eye Institute of Florida and the University of Miami School of Medicine Nasser Ibrahim Al-Rashid chair in ophthalmic plastic, orbital surgery and oncology.
“The intent is that in one process we can have a finalized product without first producing it, then have an artist tediously putting in the shades and color on the surface of it to match the other side,” Dr. Tse says.
Over the next year, the researchers plan to fine-tune the process to ensure accurate reproducibility on a larger scale and take steps to make the printer widely available.
While it’s difficult to project the future role of 3D printing in ophthalmology, Dr. Tse says the technology creates tremendous opportunities, particularly in oculoplastics.
“We are just embarking on something that’s very new,” he says. “I think the potential is really limitless.”
Recognize when to replace your EHR |
|
A web poll from IT research firm KLAS shows by 2016, nearly half of large hospitals will replace their current EHR systems. With so many systems available, practices should think about their specific priorities. Here are five signs it may be time to trade up for a new EHR system.
1. Not designed for iPad
2. Off-Shoring Customer Support
3. Not ICD-10 Ready
4. Not certified for 2014 Meaningful Use
5. Server-Based
— Dr. Sol Lizerbram, co-founder of HealthFusion, creators of MediTouch.
Ophthalmologists’ Salary Stats
| Medscape 2011 survey of medical specialists | Medscape 2014 survey of medical specialists |
These are 2010 salaries.

|
These are 2013 salaries.

|
|

|
| • Kane L. Medscape Ophthalmologist Compensation Report: 2011 Results. Medscape.
Published Feb 2011. Available at: http://www.medscape.com/features/slideshow/compensation/2011/ophthalmology.
• Peckham C. Medscape Ophthalmologist Compensation Report 2014. Medscape. Published April 15, 2014. Available at: http://www.medscape.com/features/slideshow/compensation/2014/ophthalmology. |
|
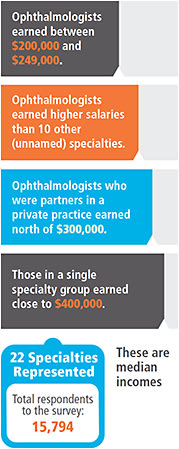
Derek Preece, who manages the American Academy of Ophthalmology’s salary benchmarking survey every year, says the salaries listed in the Medscape surveys of medical specialists (to the right) were not far from those he sees. That said, had Medscape wanted to get even more granular, it would have asked for, and listed, ophthalmology’s various subspecialties. (Medscape did acknowledge the subspecialties and the higher salaried pecking order in its notes about the survey.) The retinal surgeons make more than general [practitioners], pediatric ophthalmologists make less,” but they are combined here, says Mr. Preece, who is also a principal and executive consultant with BSM Consulting.
The recovery from the Great Recession is seen in the 2014 salary numbers. “When the recession hit, [practices] slimmed down on staff, paying attention to business aspects,” Mr. Preece says. And, like practices, patients pay took a hit in 2010 – patients didn’t have the extra cash to pay for premium lenses.
The trend, says Mr. Preece, is bending upwards.
See OM’s physician compensation model story, p. 56.









