Amniotic Membrane: Themes and Variations
Mother’s own remedy for ocular surface recovery, repair and reconstruction
BY KENNETH R. KENYON, MD AND HELENE LAM, MD
Having now nearly two decades of renewed application in ophthalmology, amniotic membrane (AM) has become established as standard therapy for numerous ocular surface disorders.
This inner layer of the placenta is predominantly an avascular connective tissue, composed of a thick basement membrane, overlying a stromal matrix of collagens plus fibronectin and laminin, as well as multiple bio-active components. These latter trophic growth stimulators, including epidermal and keratocyte growth factors, plus anti-inflammatory cytokines, act in concert to promote reepithelialization, reduce inflammation and inhibit neovascularization. The membrane is harvested during Caesarean section, then dissected and preserved by various methods.1,2
In the United States, two types of preservation methods have become commercially available: a cryopreserved version (BioTissue) and a dehydrated preparation (IOP Ophthalmics). Furthermore, both products are available in a range of thicknesses (35 to 100+ microns for dehydrated AM versus 100 to 400 microns for cryopreserved AM), depending on whether its intended application is as a biological bandage, as a permanent surface graft or as tectonic support (Figure 1). With respect to bioactivity following preservation, both cryopreserved and dehydrated AM demonstrate several active cytokines, although only the former retains high molecular weight hyaluronan (heavy chain hyaluronic acid) known for anti-inflammatory and anti-scarring effects.2 Interestingly, both preparations appear to exert comparable efficacy in their various clinical applications.3
AM is both biocompatible with the ocular surface as well as “user friendly”, because it’s easily manipulated, shaped and surgically fixated with either sutures (10-0 nylon or Vicryl) and/or fibrin tissue adhesive (Tisseel, Baxter or Evicel, Ethicon 360). Alternatively, self-retaining devices have also been devised, specifically the AmbioDisk (IOP Ophthalmics) and ProKera (BioTissue), which can be directly applied in a non-surgical office setting.

Figure 1. Amniotic membrane preparations available in the United States include the cryopreserved Amniograft (top left) and ProKera AM adhesed to PMMA ring (bottom left) for non-surgical insertion (Bio-Tissue), as well as the dehydrated AmbioDry2 and Ambio5 forms (top right), plus the selfretaining AmbioDisk (IOP Ophthalmics, bottom right).
The numerous variations of the AM theme have resulted in “different strokes for different folks” techniques, including:
• Biologic bandage: a large diameter (approximately 15 mm) self-retaining AM disc (either ProKera or AmbioDisk) is easily inserted and subsequently removed or replaced. The ProKera AM is attached to a symblepharon ring, whereas the AmbioDisk is held in place with a bandage contact lens. Both work to promote healing of extensive corneal epithelial defects (e.g., acute chemical burn).
• Overlay graft: a large diameter AM applied with basement membrane surface externalized to facilitate extensive epithelialization and incorporation into the host tissue (e.g., limbal stem cell graft)4
• Inlay graft: small AM segment(s) in one or multiple layers, sized to fill a sterile corneal ulcer, thereby replacing the deficient stromal connective tissue as well as stimulating re-epithelialization (e.g., herpetic stromalysis)5
• Combined inlay + overlay grafts: for both deep ulceration and extensive epithelial defects, AM inlays to replace stroma plus AM overlay to promote and protect epithelial recovery (e.g., neurotrophic keratopathy with stromalysis)6
The potential indications for AM use are nearly innumerable but can be segmented into two broad areas:
Restoration of the corneal surface
• Acute ocular surface inflammation/injury: chemical or thermal burn, Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN)
• Persistent epithelial defects: herpes, diabetes, numerous other
• Post-infectious ulcers: bacterial, viral, fungal, protozoal limbal stem cell (LSC) deficiency: adjunctive to limbal allo- and auto-graft, or ex vivo expansion graft
• High risk keratoplasty: neurotrophic keratopathy
• Recurrent epithelial erosion: post-trauma, anterior basement membrane dystrophy
• Superficial keratectomy: Salzmann degeneration, band keratopathy
• Bullous keratopathy if keratoplasty not indicated
• Filamentary keratitis, keratitis sicca
• Exposure keratopathy
Reconstruction of the conjunctival surface
• Acute ocular surface inflammation/injury: chemical or thermal burn, SJS, TEN
• Pterygium
• Surgical removal of large conjunctival lesions: tumors, scars, conjunctivochalasis
• Fornix reconstruction, symblepharon
• Glaucoma filter revision/repair, tube shunt exposure
• Scleral thinning
Given the relative rarity plus the variability of severity of these multiple conditions, it is nearly impossible to mount rigorous clinical trials comparing AM with other treatment options, except for the specific example of AM versus conjunctival auto-graft for pterygia, both of which have comparably low recurrence rates.7 Hence, within the scope of this brief review, we focus on the specific indications for which we personally believe AM use to be of vital importance as follows:
Acute chemical/thermal burn, SJS, TEN: The acute phase of these conditions involves diffuse ocular surface inflammation and extensive epithelial destruction, frequently including the LSCs. Hence the suppression of inflammation plus preservation of remaining LSCs and promotion of epithelial resurfacing are paramount and all within the scope of AM capabilities. Self-retaining AM (ProKera or AmbioDisc) can be readily inserted and periodically replaced until the surface has stabilized. Large AM overlay grafts extending into the fornices may reduce the loss of conjunctival goblet cells and the formation of symblepharon (Figure 2). The prognosis for primary recovery as well as the prognosis for subsequent LSC replacement and/or keratoplasty is thereby improved.8,9
Persistent epithelial defects with or without sterile stromalysis: Whether as a consequence of LSC deficiency (e.g., chemical injury), neurotrophic keratopathy (e.g., herpes simplex or zoster) or corneal exposure (e.g., incomplete blink), a non-healing corneal epithelial defect, often accompanied by sterile stromalysis is an ominous development, given the risk of perforation. Combinations of single- or multi-layer AM inlay (to replace deficient stroma) plus AM overlay (to protect and promote re-epithelialization) are superior to therapeutic contact lenses and/or lid closure alone (Figures 3 and 4).6-10
High-risk lamellar or penetrating keratoplasty: In the settings of multiple prior keratoplasties, LSC efficiency and/or neurotrophic keratopathy, the development of ocular surface failure with persistent epithelial defects leading to stromal neovascularization and loss of clarity, with or without immunologic rejection, pose the greatest post-operative risk for subsequent keratoplasty. To diminish such risks, the concomitant strategy of lamellar keratoplasty (if corneal endothelial function remains adequate), large diameter AM graft and minimal lateral tarsorrhaphy (3 mm), has improved visual and anatomical success rate to 90% (Figure 5).11,12
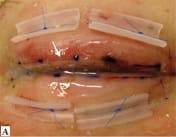
Figure 2. Amniotic membrane for fornix reconstruction in acute Stevens-Johnson syndrome (SJS). Extensive superior and inferior symblepharon are lysed. Large AM sheets, stromal side down, are secured to the external eyelid skin within 2 mm of the lash line using 8-0 nylon sutures. The AM is wrapped into the fornix and secured with 6-0 polypropylene mattress sutures through the lid over silicone bolsters. (Reprinted from Ophthalmology; vol. 118, Gregory DG, Treatment of acute Stevens-Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases., pp. 908-914, 2011, with permission from Elsevier.
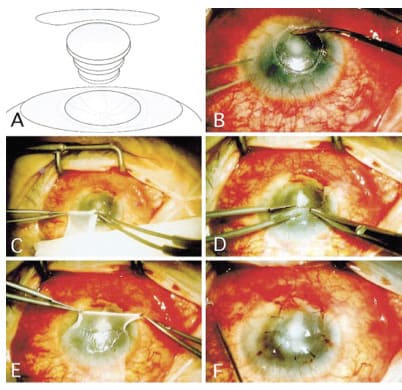
Figure 3. AM for persistent epithelial defect (PED). If accompanied by sterile stromalysis, multiple AM inlays fill the stromal defect with AM overlay to promote re-epithelialization (A). Management of PED following keratoplasty involve debridement with cellulose microsponges (B), removal of AM graft from carrier (C), position and configuration of AM overlay (D and E), secure large diameter AM overlay to corneal surface with sutures or fibrin adhesive. A bandage, soft contact and/or lateral tarsorrhaphy may also be utilized. (Reprinted from Ophthalmology; vol. 106, Kruse FE, Rohrschneider K, Volcker HE et al., Multilayer amniotic membrane transplantation for reconstructions of deep corneal ulcers. 1504-1511, 1999, with permission from Elsevier.)
Limbal stem cell transplantation: Whether as an adjunct to classic limbal autograft4 or allograft transplantation or as the substrate for ex vivo LSC expansion and transplantation,13,14 AM overlay grafts promote and stabilize corneal re-epithelialization.
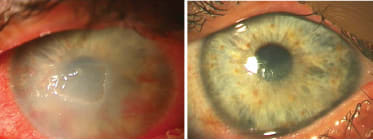
Figure 4. AM for PED with stromalysis in a 32-year-old male with neurotrophic keratopathy secondary to neurofibromatosis. Left: Central corneal PED and early stromalysis reduce visual acuity to counting fingers with conjunctival inflammation, stromal diffuse haze and active neovascularization, despite use of soft contact lens (SCL), tarsorrhaphy and gold weight lid implant. Right: Following AM overlay and mini-lateral tarsorrhaphy, VA improves to 20/60, and the eye has no inflammation with healed and stable corneal epithelium, clear stroma and regressed neovascularization without need for therapeutic SCL.
Fornix reconstruction: For the late stage management of symblepharon following chemical injury, SJS, TEN and some ocular cicatricial pemphigoid cases, large area AM grafts to cover the deepithelialized bulbar and tarsal conjunctival surfaces and to restore the fornices are superior to artificial membranes (Figure 6).15 For extensive limbal and conjunctival defects created by excision of large ocular surface tumors (e.g. squamous carcinoma or melanoma),16 AM grafts again promote ocular surface healing and inhibit symblepharon formation. AM is more cosmetically attractive than oral mucous membrane grafts and its intrinsic transparency also facilitates monitoring of potential tumor recurrence (Figure 7).
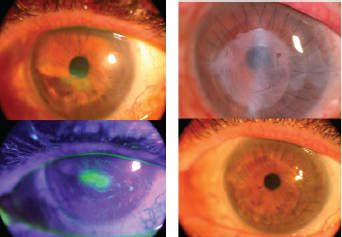
Figure 5. AM for hisk keratoplasty. Following herpes zoster, neurotrophic keratopathy with PED, stromal thinning and scarring reduced visual acuity to 20/400 (left top and bottom). A deep anterior lamellar keratoplasty with AM overlay and lateral mini-tarsorrhapy (top right, with AM remnants still present) improved visual acuity to 20/30 with corneal epithelial stability allowing tarsorrhaphy release (bottom right).
Summary
AM is remarkable in its protean applications and benefits for ocular surface recovery, repair and reconstruction. Apart from imparting a compatible basement membrane and connective tissue matrix, its multiple trophic factors promote re-epithelialization and suppress inflammation, while minimizing neovascularization and scarring. Hence clinical management of persistent corneal epithelial defects, LSC deficiency, high risk keratoplasty and numerous other ocular surface challenges are greatly enhanced through the custom tailored application of the several product variations of AM via various surgical techniques. The increasing uses of AM as a substrate for cultured ocular surface amplification as well as the isolation and direct topical application of its bioactive factors suggest that the ocular and extraocular potential of AM remains promising. ■

Figure 6. AM for symblepharon release and fornix reconstruction. Left: Scar tissue extends from the palpebal lid margin to the bulbar perilimbal area. Right: Subconjunctival fibrous tissue is dissected, residual conjunctiva is reflected into the fornix and secured to the palpebral surface. A large AM graft is sutured to the conjunctival margin and episclera, and the fornix is reformed as two double-armed 5-0 nylon mattress sutures are passed through the lid and secured over the lower lid skin (Reprinted from Ophthalmology; vol. 110, Solomon A, Espana EM, Tseng SCG, et al. Amniotic membrane transplantation for reconstruction of the conjunctival fornices. 93-100, 2003, with permission from Elsevier.)
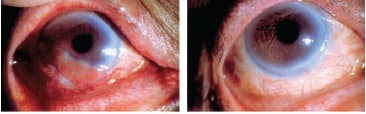
Figure 7. AM for ocular surface reconstruction. Left: A large limbal squamous cell carcinoma has extended onto the corneal surface, requiring superficial keratectomy, wide conjunctival excision and limbal cryotherapy, plus large single AM layer secured with sutures and fibrin adhesive. Right: The conjunctival, limbal and corneal epithelial defects have healed with excellent cosmesis and without scarring or tumor recurrence. (Reproduced with permission from the Chang Gung Medical Journal. Yeh LK, Lin HC, Ma DH. Amniotic membrane grafts following excision of corneal and conjunctival intraepithelial neoplasia. 2003;10:737-744.)
References
1. Meller D, Pauklin M, Thomasen H, et al. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int 2011;108:243-248.
2. Tseng SC, Espana EM, Kawatika T, et al. How does amniotic membrane work? Ocul Surf 2004;2:177-187.
3. Kenyon KR. Amniotic membrane: Mother’s own remedy for ocular surface disease. Cornea 2005;24:639-642.
4. Kenyon KR, Tseng CSG. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989;96:709-723.
5. Kruse FE, Rohrschneider K, Volcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999;106:1504-1510.
6. Seitz B. Amniotic membrane transplantation. An indispensable therapy option for persistent corneal epithelial defects. Ophthalmology 2007;104:1075-1079.
7. Zheng K, Cai J, Jhannji V, et al. Comparision of pterygium recurrence rates after limbal conjunctival autograft transplantation and other techniques: metaanalysis. Cornea 2012;31:1422-1427.
8. Meller D, Pires RT, Mack RJ et al. Amniotic membanae transplantation for acute chemical or thermal burns. Ophthalmology 2000;107:980-989.
9. Kherikhah A, Johnson DA, Paranjpe DR et al. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol 2008;126:1059-1066.
10. Solomon A, Meller D, Prabhasawat P, et al. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles and deep ulcers. Ophthalmology 2002;109:694-703.
11. Kenyon KR, Langston DP. Therapeutic advances in neurotrophic keratopathy. Ophthalmology Management 2012; 15-19.
12. Seitz B, Das S, Sauer R, et al. Simultaneous amniotic membrane patch in high-risk keratoplasty. Cornea 2011;30:269-272.
13. Grueterich M, Espana EM, Tseng SC. E vivo expansion of limbal epithelial stem cells: Amniotic membrane serving as a stem cell niche. Surv Ophthalmol 2003;48:631-646.
14. Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med 2000;343:86-93.
15. Gregory DG. Treatment of acute Stevens-Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. Ophthalmology 2011;86:640-645.
16. Espana EM, Prabhasawat P, Grueterich M, et al. Amniotic membrane transplantation for reconstruction after excision of large ocular surface neoplasias. Br J Ophthalmol. 2002;86:640-645.
|
Kenneth R. Kenyon, MD, is Clinical Professor of Ophthalmology, Tufts University School of Medicine, Lecturer in Ophthalmology, at Harvard Medical School, Clinical Professor of Ophthalmology, New England College of Optometry and Senior Clinical Scientist, Schepens Eye Research Institute. He can be reached at kenrkenyon@cs.com.
Helene Lam, MD, is a Comprehensive Ophthalmology, Cornea and External Diseases at Harvard Vanguard Medical Associates. She can be reached at Helene_Lam@vmed.org.
|
Financial disclosures: The authors have no direct financial interest in any product discussed in this review. Dr. Kenyon is a share holder of Tissue Tech, Inc and of KEERA, s.r.l, developer of AMX, a related AM-derived product.










