A 5-step approach to diagnosing uveitis
Assume an infectious culprit, then proceed to differentiate the cause.
By Eric K. Chin, MD, and Daniel F. Kiernan, MD

|
Eric K. Chin, MD, is an ophthalmology resident at the University of California Davis Eye Center, Sacramento. He will be starting a two-year surgical vitreoretinal fellowship at the University of Iowa next month. |

|
Daniel F. Kiernan, MD, is a vitreoretinal surgeon at Ophthalmic Consultants of Long Island, Rockville Centre, N.Y. He has published dozens of peer-reviewed and non-peer reviewed articles. He is also an active clinical investigator researching both non-invasive and surgical approaches for the diagnosis and treatment of sight debilitating retinal diseases. |
Diagnosing uveitis can be a challenge. Many signs and symptoms overlap with other disease entities, and patient presentations can vary depending on the cause of the infection. In fact, clinicians often never find the exact etiology; the most common cause they give for uveitis is idiopathic anterior uveitis.1
This article reviews a stepwise approach to conducting the differential diagnosis of infectious uveitis and highlights the more common causes. Infectious posterior uveitis has the broadest differential diagnosis compared to other areas of the eye, and in children infections are the most common cause of posterior uveitis.2,3
One good rule of thumb when considering the work-up and management of uveitis is to always assume an infectious cause. Infections respond well to antibiotic or antiviral therapy, and we can proceed safely to treat the uveitis with corticosteroid or immunosuppressive therapy when we rule them out early.

1 Macular scar with adjacent reactivation of toxoplasmosis retinitis. Toxoplasmosis is the most common etiology of posterior uveitis in immunocompetent patients.
| Getting familiar with TORCHES |
|---|
|
The mnemonic “TORCHES”, typically used to describe perinatal infections, can be applied to help remember the more common causes of infectious posterior uveitis: toxoplasmosis, toxocariasis, tuberculosis, cytomegalovirus, herpes simplex/zoster and syphilis. Consider “other” (“O”) less common causes such as rubella (“R”), Epstein-Barr virus (“E”), bartonella, Lyme disease, West Nile virus, chikungunya and other HIV-related eye diseases in endemic areas or when risk factors are present. Toxoplasmosis This is most common infectious etiology of posterior uveitis in immunocompetent patients (whereas Bechet’s uveitis is the most common immunologic or non-infectious etiology).16 Bilateral macular scars can be seen in congenital disease, and active disease adjacent to a scar is seen with reactivation of previous infection (Figure 1). Retinitis without scarring is more suggestive of acquired disease. Clinical appearance: Active lesions appear as an area of focal retinochoroiditis with either mild or severe overlying vitritis.17 Most cases of toxoplasmosis in the immunocompetent host are subclinical or benign.18,19 One should make a distinction between congenital and acquired toxoplasmosis. Congenital lesions include asymptomatic punched-out macular cicatricial lesions with a central necrotic zone (10% with ocular lesions have no evidence of disease in other organs). Recurrent lesions of congenital disease often develop at the borders of the old toxoplasma scars (“satellite lesions”). Acquired disease may present with decreased vision or floaters and a characteristic “headlight-in-the-fog” appearance. The initial lesion starts in the superficial retina, gradually involving the full-thickness retina, adjacent choroid, vitreous and even sclera. A yellowish white or gray exudative lesion shows ill-defined borders because of surrounding retinal edema. Toxocariasis Accidental ingestion of larvae of the dog roundworm Toxocara canis or the cat roundworm Toxocara cati causes toxocariasis. Children with pica in close contact with puppies are particularly vulnerable. Infections in humans result in focal granulomatous reaction in many organs including the eye. Clinical appearance: Manifestations in order of decreasing likelihood include granulomas in the peripheral retina and vitreous, posterior pole granuloma, chronic endophthalmitis, or optic nerve or anterior segment involvement. Granulomas with tractional retinal detachment or a chronic endophthalmitis-like picture impairing visualization is typical. Early lesions may be difficult to visualize due to intense vitritis. Chronic masses may have significant atrophy and RPE hyperplasia. Peripheral eosinophilia is not associated with ocular toxocariasis.20 Tuberculosis TB can cause an anterior granulomatous uveitis,21 mild to moderate vitritis and multifocal choroiditis. Ocular TB can either be primary or secondary where the organisms spread to the eye via the blood. Clinical appearance: Disseminated choroiditis is the most common presentation of tuberculous uveitis. Choroidal tubercles may be one of the earliest signs of disseminated disease. Lesions may vary from few in number to several hundred. Lesions range in size from 0.5 to 3.0 mm in diameter, and may vary in both size and elevation within the same eye. They are deep in the choroid, appear yellow, white or gray, and are fairly well circumscribed.
2 Fundus photograph montage showing progressive retinitis with retinal vasculitis.
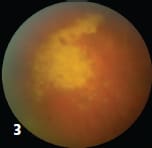
3 Ganciclovir-resistant retinitis one week after ganciclovir treatment.
4 Fluorescein angiography showing vascular occlusion and peripheral nonperfusion.
5 Acute retinal necrosis, seen here, is the classic presentation of herpetic viruses. Herpetic posterior uveitis often results in serious complications such as retinal detachment and proliferative vitreoretinopathy. In most cases, lesions present in the posterior pole, where a single tubercle (or focal choroiditis) may also occur.22 The mass is typically elevated and may be accompanied by an overlying serous retinal detachment.23 Subretinal abscesses, which can be either single or multiple, may complicate a choroidal tubercle. Cytomegalovirus CMV retinitis is an opportunistic infection classically seen in HIV-infected patients with low CD4 cell counts. It can also occur in heart or bone marrow transplant patients on immunosuppressant therapy. Clinical appearance: Findings include fine cell debris on the corneal endothelium, mild vitritis and yellow-white slowly progressive retinitis in any area of the retina, with retinal vasculitis (predominantly involving retinal arteries) and hemorrhages (Figures 2 and 3). CMV can involve the posterior pole initially and mimic cotton wool spots of HIV retinopathy in early stages. It may also lead to peripheral retinal nonperfusion (Figure 4), ischemia, retina neovascularization, vitreous hemorrhage and retinal detachment. Herpetic viruses Among the most common causes of infectious uveitis, herpetic infections can affect both healthy and immunocompromised individuals. Most cases of herpetic posterior uveitis are acute and fulminant, often resulting in serious complications such as retinal detachment and proliferative vitreoretinopathy. Clinical appearance: Acute retinal necrosis is the classic presentation of herpetic viruses. The characteristic triad of moderate to severe vitritis, arteritis/periphlebitis and confluent peripheral retinal necrosis is diagnostic (Figure 5). Although the posterior pole is not typically affected early in the disease process, it can be involved primarily in necrotizing herpetic retinopathies. Progressive outer retinal necrosis is another manifestation of herpes. Necrotizing retinitis, confluent areas of outer retinal whitening involving the posterior pole and sparing of retinal vessels at the early stage (“cracked mud appearance”) is diagnostic. Progressive outer retinal necrosis is frequently bilateral, without significant vitritis, and occurs exclusively in immunocompromised states. It has been associated with rapid development of rhegmatogenous retinal detachment or optic atrophy. Syphilis Besides sexual contact, syphilis can be spread via blood transfusions and across the placenta to the fetus. Clinical appearance: Clinical findings are widespread, hence its nickname “the great masquerader” or “great imitator,”and include anterior uveitis (either granulomatous or nongranulomatous), salt-pepper retinopathy, vitritis, retinitis, multifocal choroiditis, neuroretinitis, optic neuritis, papilledema and optic perineuritis.24 Syphilis can present with placoid choroidal lesions and can mimic acute posterior multifocal placoid pigment epitheliopathy.9 Syphilitic uveitis can be the first presentation of the systemic disease in both immunocompetent and immunocompromised individuals.25 |
STEP 1: GATHER THE HISTORY
A thorough history is critical in evaluating patients with uveitis. Besides the general medical history, the history should comprise systemic diseases, travel history, social history, medications and family history.
Age alone can narrow the possibilities. Congenital disease transmissible from the mother to fetus has been seen with toxoplasmosis, syphilis and cytomegalovirus (CMV). Toxoplasmosis and toxocariasis are the most common causes of infectious uveitis in children. Consider herpes simplex in middle-aged adults4 and varicella zoster (VZV) in elderly patients.5
Other markers
The duration of symptoms is also important. One may differentiate rapidly progressive conditions, such as viral retinal necrosis, from toxoplasmosis and Behcet’s disease, which tend to present within one to two weeks after symptoms first appear. This contrasts to other diseases that progress slower, such as mycotic or nematode infections.
Gathering information about environmental triggers may also help. Exposure to cats or under-cooked meat may point to toxoplasmosis. Dog exposure may point to toxocariasis, and tick bites to Lyme disease. If a patient is immunocompetent, acute retinal necrosis (ARN) secondary to herpes may be more likely than other viral infections.
An immunocompromised state may predispose a patient to VZV6, CMV or endogenous endophthalmitis. Besides HIV, immunocompromised individuals may be on long-term antibiotics, steroids or immunosuppressives, or have indwelling catheters. Timing of symptoms also plays an important role. Any IV drug use should also be considered a risk factor.
STEP 2: DEFINE THE PRIMARY LOCATION
Uveitis is defined as inflammation of uveal tissue. This includes the iris, ciliary body and choroid. However, “spillover” inflammation, when severe, can confound the picture. Neighboring structures, such as the cornea, retina and optic nerve, may also become involved.7 Anterior disease is the most common form of uveitis, accounting for 50% to 60% of all such cases in tertiary care centers.8
Ruling out herpetic causes
In all cases of suspected viral panuveitis, careful anterior segment examination to look for diagnostic clues suggestive of a herpetic cause may be useful. Corneal sensation is a useful test along with careful inspection of the iris for subtle transillumination defects, which may suggest underlying herpetic disease.
Laterality also helps narrow the diagnosis. Bilateral disease occurs in up to 35% of patients with ARN, usually within six weeks.9 Bilateral disease occurs in more than 70% of cases of progressive outer retinal necrosis secondary to VZV.9 Toxoplasmosis and toxocariasis typically present with unilateral disease in an overwhelming majority of cases.3
STEP 3: CHARACTERIZE THE LESION
Look at the primary lesion. Is it focal or diffuse? The classic “headlight in the fog” or focal chorioretinitis with overlying vitritis adjacent to a chorioretinal scar may be acquired toxoplasmosis. These lesions often remain active for up to 16 weeks and spontaneously resolve, leaving a hyperpigmented scar. Note that classic toxoplasmosis retinitis involves the inner retina with intense vitritis. Conversely, the outer retinal form of toxoplasmosis has minimal vitritis because it involves the deeper retinal layers with less spillover of inflammatory cells into the vitreous cavity.10
Do not confuse these lesions with choroidal tubercles (small, yellow nodules with indistinct borders) or choroidal tuberculomas (larger, solitary, tumor-like masses that may grow vertically or spread diffusely in the choroid), which are usually due to tuberculosis. A focal, well-demarcated area of retinal necrosis, occlusive vasculopathy and arteriolar involvement, with an intense vitreous and anterior chamber reaction, may suggest ARN. A large area of “brush fire” hemorrhage and white, edematous or necrotic area may be the classic “pizza pie” appearance in CMV retinitis.
STEP 4: EXAMINE OUTSIDE THE EYE
After examining both eyes, always evaluate the patient for systemic signs and symptoms outside the eye. Syphilis, for example, may involve the bone, central nervous system, liver, lung or spleen. Patients may present with fever, rash, hepatosplenomegaly, pneumonia, anemia and/or generalized lymphadenopathy. In CMV, young patients may have microcephaly, encephalitis, deafness and psychomotor retardation. In Lyme disease, one may have a “bulls-eye” rash, arthritis, fatigue or other cardiac and neurologic findings.
STEP 5: ORDER ANCILLARY TESTING
Lastly, ancillary testing can confirm or rule out certain infectious etiologies. In acute posterior uveitis, an exact clinical diagnosis is sometimes not possible due to dense vitreal infiltration for which these tests are exceedingly helpful.
What tests to order
For syphilis, both a non-treponemal tests (RPR or VDRL) and treponemal tests (FTA-ABS or TPPA) are indicated. Remember, treponemal tests cannot quantify disease or response to therapy and they usually remain positive for life (as in the case of congenital disease) unlike the non-treponemal tests. A purified protein derivative or quantiferon-TB gold assay is helpful for tuberculosis.
Specular and in vivo confocal microscopy, polymerase chain reaction and pathogen-specific antibody production may also give the most definitive cause if intraocular specimens can be obtained.12,13,14,15
Ancillary testing is important because resurgent arthropod vector-borne diseases can cause systemic morbidity and death. OM
REFERENCES
1. Yanoff M, Duker JS. Augsburger JJ. Ophthalmology. 2nd ed. St. Louis, MO: Mosby;2004.
2. Markomichelakis NN, Chatzistefanou KI, Papaefthymiou I, et al. Infectious uveitis in children. Ophthalmology 2009;116:1588.
3. Garweg JG, Tappeiner C. Differential diagnosis in infectious posterior uveitis. Klin Monbl Augenheilkd. 2011;228:268-272.
4. Ganatra JB, Chandler D, Santos C, et al. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol 2000;129:166-172.
5. Chatzistefnou K, Markomichelakis NN, Christen W, et al. Characteristics of uveitis presenting for the first time in the elderly. Ophthalmology 1998;105:347-352.
6. Kuppermann BD, Quiceno JI, Wiley C, et al. Clinical and histopathologic study of varicella zoster virus retinitis. Am J Ophthalmol 1994;118:589-600.
7. Duane TD. Clinical Ophthalmology, Rev ed. Hagerstown, MD: Medical Dept, Harper & Row; 1978.
8. Albert DM, Jakobiec FA. Principles and Practice of Ophthalmology. 2nd ed. Philadelphia: W.B. Saunders Co.;2000.
9. Mandelcorn ED. Infectious causes of posterior uveitis. Can J Ophthalmol 2013;48:31-39.
10. Doft BH, Gass DM. Punctate outer retinal toxoplasmosis. Arch Ophhthalmol. 1985;103:1332-1336.
11. Gupta V, Gupta A, Rao NA. Intraocular tuberculosis – an update. Surv Ophthalmol. 2007;52:561-587.
12. Mahendradas P, Sherry R, Narayana KM, Sherry BK. In vivo confocal microscopy of keratic precipitates in infectious versus noninfectious uveitis. Ophthalmology 2010;117:373-380.
13. Sudharshan S, Banesh SK, Biswas J. Current approach in the diagnosis and management of posterior uveitis. Indian J Ophthalmol. 2010;58:29-43.
14. Tran TH, Rozenberg F, Cassoux N, et al. Polymerase chain reaction analysis of aqueous humour samples in necrotizing retinitis. Br J Ophthalmol 2003;87:79-83.
15. Harper TW, Miller D, Schiffman JC, et al. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am J Ophthalmol 2009;147:140-147.
16. Cunningham ET Jr. Uveitis in children. Ocul Immunol Inflamm. 2000;8:251-261.
17. Dhoot DS, Martin DF, Srivastava SK. Pediatric infectious posterior uveitis. Int Ophthal Clin 2011;51:113-128.
18. Bonfioli AA, Orefice F. Toxoplasmosis. Semin Ophthalmol. 2005;20:129-141.
19. Palanisamy M, Madhavan B, Balasundaram MB, Andavar R. Venkatapathy N. Outbreak of ocular toxoplasmosis in Coimbatore, India. Indian J Ophthalmol. 2006;54:129-131.
20. Vitale AT, Foster CS. Uveitis affecting infants and children: infectious cuases. In: Hartnett ME, Trese M, Capone A Jr, et al, eds. Pediatric Retina. Philadelphia: Lippincott, Williams & Wilkins; 2005:249-289.
21. Thomson MJ, Albert DM. Ocular tuberculosis. Arch Ophthalmol 2005;123:844-849.
22. Cangemi FE, Freidman AH, Osephberg R. Tuberculoma of the choroid. Ophthalmology 1980;87:252-258.
23. Bernstein DM, Gentile RC, McCormick SA, Walsh JB. Primary choroidal tuberculoma. Arch Ophthalmol. 1997;115:430-431.
24. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standarization of Uveitis Nomenclature (SUN) Working Group: Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol 2005;140:509-516.
25. Hong MC, Sheu Sj, Wu TT, Chuang CT. Ocular uveitis as the initial presentation of syphilis. J Chin med Assoc 2007;70:274-280.